RFID began many years ago for tracking livestock, but back then it was referred to as Tags and had limited data. Destron Fearing has two kinds of tags, one that is attached to the farm animal, and one that is injected into the animal, whether it be a fish, cow, pig, cat or dog. “Life chip” is one of the names the injectable RFID is known by today. In Canada most SPCA animal shelters chip the animals before the animal is adopted. Some animals had the chip removed due to CANCER caused by the chip. (see article below)
The well known names “Verichip” (2001) and “Digital Angel” (2000) were the next generation of RFID, but as the idea of this technology was discussed in the media, people had growing concerns over privacy and health effects.

In addition, the Cell Phone industry was already under critizism from users getting brain tumours, which may have set back the RFID implant industry in the early 2000’s. Since that time the company has changed names and is now under the brand names Positive ID (PSID) and Veri Teq, mostly focusing on devices to assist various medical fields, however they are likely still considering the implant rfid for future usage at some point.
Even with the radiation risks, some believe it will be implemented via the Obamacare Health plan as shown in videos below.
RFID tags are classed based upon a frequency or some folks call a read range
– Low Freq Range: 50cm. Use: Good for tagging pets/people
– High Freq Range: 3m. Use: Access control for buildings, doors
– Ultra high Freq Range: 9m. Use: Pallets, Boxes for inventory control
– Microwave Range: >10m. Use: Vehicle tracking
Radio-frequency identification
https://en.wikipedia.org/wiki/Radio-frequency_identification
https://www.facebook.com/pages/Radio-frequency-identification/108144822541070?fref=ts#
https://en.wikipedia.org/wiki/Digital_Angel
https://en.wikipedia.org/wiki/VeriChip
https://en.wikipedia.org/wiki/PositiveID

Patents
July 31, 1997 Paul A. Gargano, David Hunt Gilmore, Frank A. Pace, Lee Weinstein
transmitters operating at a frequency of 460 MHz
May 13, 1997 Paul A. Gargano, David Hunt Gilmore, Frank A. Pace, Lee Weinstein
patent: WO1997027499A1 (5629678)
radio frequency is in the 460 MHz band
RFID Journal says : “Verichip is said by media coverage to be operating at 134 kHZ passive tag “
Spetrum IEEE says : “The chip consists primarily of a coil of wire that acts as an antenna and a microchip capable of generating a radio signal that encodes 128 bits of information and is readable from, at most, centimeters away. The reading device emits a magnetic field that oscillates at a frequency of 134 kilohertz. The reader and the chip’s antenna basically form a transformer, turning the oscillating magnetic field into current in the implant.”
It is interesting to see the change in frequency from 460 MHZ to 134 kHZ. Spectum IEEE thinks its only going to send data a few centimetres, where below, Digital Angel and Verichip were thinking longer distances – to cellular antennas and satellites.
Digital Angel Website ( 2000 )
Digital Angel Website ( 2000 )
APPLIED DIGITAL SOLUTIONS INTRODUCES VERICHIP™, A MINIATURIZED, IMPLANTABLE IDENTIFICATION DEVICE WITH A VARIETY OF MEDICAL, SECURITY AND EMERGENCY APPLICATIONS |
PALM BEACH, FL — December 19, 2001 – – Applied Digital Solutions, Inc. (Nasdaq: ADSX), an advanced digital technology development company, announced today that it has developed a miniaturized, implantable identification chip — called VeriChip™ — that can be used in a variety of medical, security and emergency applications.
How VeriChip Works
VeriChip is an implantable, 12mm by 2.1mm radio frequency device about the size of the point of a typical ballpoint pen. Each VeriChip will contain a unique identification number and other critical data. Utilizing an external scanner, radio frequency energy passes through the skin energizing the dormant VeriChip, which then emits a radio frequency signal transmitting the identification number and other data contained in the VeriChip. The scanner will display the identification number, but the VeriChip data can also be transmitted, via telephone or the Internet, to an FDA compliant, secure data-storage site. It will then be accessible by authorized personnel. Inserting the VeriChip device is a simple procedure performed in an outpatient, office setting. It requires only local anesthesia, a tiny incision and perhaps a small adhesive bandage. Sutures are not necessary.
Medical Device Identification
Hundreds of thousands of medical devices are surgically implanted into patients every year. Examples of these life-saving and life-enhancing devices include pacemakers, artificial joints, orthopedic hardware, heart valves, and medication pumps. After insertion, these devices often require adjustment, repair, replacement, or even recall. VeriChip, inserted subdermally just above the implanted medical device, provides patients, medical providers, and manufacturers with a rapid, secure and non-invasive method for obtaining medically critical information about the device. VeriChip is a ready source of data about the patient’s name and condition as well as the medical device’s original components, required settings and other essential parameters. Future applications may include full medical record archival/retrieval for emergency medical care.
Emergency or Security-related Identification
Personal identity verification technology has gained considerable interest recently. A great deal of focus has been trained on so-called “biometric” technologies – which identify individuals by their unique biological or physical characteristics, such as fingerprints, voiceprints, retina characteristics, and face recognition points. VeriChip, by contrast, relies on imbedded, tamper-proof, microchip technology, which allows for non-invasive access to identification, medical and other critical data. Use of advanced VeriChip technology means that the threat of theft, loss, duplication or counterfeiting of data is substantially diminished or eliminated. Specific application areas include: enhancement of present forms of identification, search and rescue, and various law enforcement and defense uses.
Commenting on the announcement, Richard J. Sullivan, Chairman and CEO of Applied Digital Solutions stated: “With VeriChip, Applied Digital has taken another significant step in developing leading-edge personal security technologies for a rapidly evolving marketplace. VeriChip joins Digital Angel™ and Thermo Life™ in our repertoire of breakthrough technologies. All of these are designed specifically to save lives, enhance personal security and improve quality of life. We’re looking forward to working with the medical community and other potential partners to bring VeriChip to market as quickly as possible.”
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
THERMO LIFE ( ADSX – Applied Digital Solutions )
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
Thermo Life in “Bio Thermo” by Destron Fearing, used in Pigs (2011)
Bio-Thermo LifeChip Review in Swine
••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
Kevin Rudd’s e-Health bill paves the way for PositiveID human implantable RFID microchips
By Greg Nikolettos Sci Tech 4/12/2010
http://www.opednews.com/articles/Kevin-Rudd-s-e-Health-bill-by-Greg-Nikolettos-100409-619.html
Grid / RFID : One Mainframe To Rule Them All – Full Version (2009)
Original Source : Parts 1 to 5 (2009)
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
We The People will not be Chipped
http://www.wethepeoplewillnotbechipped.com
SpyChips
http://www.spychips.com/what-is-rfid.html
http://www.spychips.com/about_us.html
AntiChips
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
FDA, HR 3962 – RFID Device and Obamacare – Part 1 of 2
FDA, HR 3962 – RFID Device and Obamacare – Part 2 of 2
RFID Implant company think everyone will get one
Joe Biden : Mark my words, you will rule on RFID
Bush pushes RFID
RFID Healthcare Bill – USA, July 2011
RFID Chip Required in Obama’s Health Care Bill – by Dec 31, 2017
HIPAA – Health Insurance Portability and Accountability Act
RFID in Healthcare
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
VeriMed Patient Identification System
Feb. 26, 2007 – please see also hopetotheend.com/medchip.html
“Target market for The VeriMed system consists of people who are more likely to require emergency medical care, persons with cognitive impairment, persons with chronic diseases and related conditions, and persons with implanted medical devices. CHIP believes their system will be of use for emergency personnel and first responders.
Personally, I foresee a myriad of technology issues here. If these systems are sold to healthcare plans, hospitals or individual potential patients, to hold any value whatsoever the unresponsive future patient would have to be at or taken to a facility using the database with that patient’s information. If these were purchased by facilities, they’re already going to have medical information readily available for their patients in their existing databases.
This system would only have value if a patient is brought in ‘cold’ meaning a hospital or facility would have no existing information on this patient. That is the entire purpose of reading the implanted chip. In that case, what really would be the odds that an implanted patient would just happen to end up being taken to a facility or hospital that bought the system and has access to the database linking the patients implanted chip with the patient information????
…. If the person is institutionalized, that institution will already have information readily available for this person in case of an emergency, so an implant would be of little added help. These would work and be of use only in situations in which the responders had no information on an unresponsive patient…and would require a lot of luck in matching up an implanted patient with a facility that purchased access to the database“
http://biz.yahoo.com/seekingalpha/070211/26597_id.html?.v=1
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
FDA approves computer chip for Humans
Devices could help doctors with stored medical information
http://www.nbcnews.com/id/6237364/#.VLGEEEuQYjI
updated 10/13/2004 6:38:52 PM ET
WASHINGTON — Medical milestone or privacy invasion? A tiny computer chip approved Wednesday for implantation in a patient’s arm can speed vital information about a patient’s medical history to doctors and hospitals. But critics warn that it could open new ways to imperil the confidentiality of medical records.
The Food and Drug Administration said Wednesday that Applied Digital Solutions of Delray Beach, Fla., could market the VeriChip, an implantable computer chip about the size of a grain of rice, for medical purposes.
With the pinch of a syringe, the microchip is inserted under the skin in a procedure that takes less than 20 minutes and leaves no stitches. Silently and invisibly, the dormant chip stores a code that releases patient-specific information when a scanner passes over it.
Think UPC code. The identifier, emblazoned on a food item, brings up its name and price on the cashier’s screen.
Chip’s dual uses raise alarm
The VeriChip itself contains no medical records, just codes that can be scanned, and revealed, in a doctor’s office or hospital. With that code, the health providers can unlock that portion of a secure database that holds that person’s medical information, including allergies and prior treatment. The electronic database, not the chip, would be updated with each medical visit.
The microchips have already been implanted in 1 million pets. But the chip’s possible dual use for tracking people’s movements — as well as speeding delivery of their medical information to emergency rooms — has raised alarm.
“If privacy protections aren’t built in at the outset, there could be harmful consequences for patients,” said Emily Stewart, a policy analyst at the Health Privacy Project.
The resource you are looking for might have been removed, had its name changed, or is temporarily unavailable.
To protect patient privacy, the devices should reveal only vital medical information, like blood type and allergic reactions, needed for health care workers to do their jobs, Stewart said.
An information technology guru at Detroit Medical Center, however, sees the benefits of the devices and will lobby for his center’s inclusion in a VeriChip pilot program.
“One of the big problems in health care has been the medical records situation. So much of it is still on paper,” said David Ellis, the center’s chief futurist and co-founder of the Michigan Electronic Medical Records Initiative.
‘Part of the future of medicine’
As “medically mobile” patients visit specialists for care, their records fragment on computer systems that don’t talk to each other.
“It’s part of the future of medicine to have these kinds of technologies that make life simpler for the patient,” Ellis said. Pushing for the strongest encryption algorithms to ensure hackers can’t nab medical data as information transfers from chip to reader to secure database, will help address privacy concerns, he said.
The U.S. Department of Health and Human Services on Wednesday announced $139 million in grants to help make real President Bush’s push for electronic health records for most Americans within a decade.
William A. Pierce, an HHS spokesman, could not say whether VeriChip and its accompanying secure database of medical records fit within that initiative.
“Exactly what those technologies are is still to be sorted out,” Pierce said. “It all has to respect and comport with the privacy rules.”
Applied Digital gave away scanners to a few hundred animal shelters and veterinary clinics when it first entered the pet market 15 years ago. Now, 50,000 such scanners have been sold.
To kickstart the chip’s use among humans, Applied Digital will provide $650 scanners for free at 200 of the nation’s trauma centers.
Implantation costs $150 to $200
In pets, installing the chip runs about $50. For humans, the chip implantation cost would be $150 to $200, said Angela Fulcher, an Applied Digital spokeswoman.
Fulcher could not say whether the cost of data storage and encrypted transmission of medical information would be passed to providers.
The resource you are looking for might have been removed, had its name changed, or is temporarily unavailable.
Because the VeriChip is invisible, it’s also unclear how health care workers would know which unconscious patients to scan. Company officials say if the chip use becomes routine, scanning triceps for hidden chips would become second nature at hospitals.
Ultimately, the company hopes patients who suffer from such ailments as diabetes and Alzheimer’s or who undergo complex treatments, like chemotherapy, would have chips implanted. If the procedure proves as popular for use in humans as in pets, that could mean up to 1 million chips implanted in people. So far, just 1,000 people across the globe have had the devices implanted, very few of them in the United States.
The company’s chief executive officer, Scott R. Silverman, is one of a half dozen executives who had chips implanted. Silverman said chips implanted for medical uses could also be used for security purposes, like tracking employee movement through nuclear power plants.
Such security uses are rare in the United States.
Meanwhile, the chip has been used for pure whimsy: Club hoppers in Barcelona, Spain, now use the microchip to enter a VIP area and, through links to a different database, speed payment much like a smartcard.
© 2012 The Associated Press
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
ANIMALS : “Chip Me NOT”
http://www.chipmenot.org/casehistories.htm
http://www.chipmenot.org/microchiprisks.htm
http://www.antichips.com/press-releases/chipped-pets.html
http://whyfry.org/chipped-pets-develop-fast-growing-lethal-tumors/
French Bulldog is Catalyst for Investigation of Microchip-Cancer Connection
September 8, 2007
Could a microchip implant like the VeriChip cause cancer? A French Bulldog named Léon was the catalyst for new questions about the safety of RFID implants.
One year ago, Léon’s owner contacted me with startling news. She believed that her dog’s cancerous tumor and his untimely death might have been caused by a microchip implant.
This was not just idle talk by a grieving dog owner grasping at straws to figure out why she had been robbed of her constant companion. This was a gutsy lady who refused to allow the vet to simply cremate the evidence.
This lady prefers to be known only by her first name of “Jeanne,” so the Associated Press couldn’t credit her properly as the original source for some of the explosive information in its article “Chip Implants Linked to Animal Tumors,” but I have the leeway in this forum to share the behind-the-scenes story.
Jeanne spent a small fortune trying to cure her ailing French bulldog, Léon, after he was diagnosed with cancer in 2004. When medical interventions failed and Léon passed away, she decided to hunt for the reason the fatal tumor in his body was attached to the glass-encapsulated microchip that had been injected into his neck for identification purposes.
Jeanne located a team of researchers in Italy who agreed to test tissue samples from a biopsy of Léon’s tumor to determine if the microchip was implicated in his aggressive cancer. They documented their findings in a 2006 paper entitled, “Fibrosarcoma with Typical Features of Postinjection Sarcoma at Site of Microchip Implant in a Dog: Histologic and Immunohistochemical Study.” The full text is available online at: http://www.vetpathology.org/cgi/content/full/43/4/545.
Since Léon’s suspicious cancer was not enough evidence to prove microchip implants were a threat, Jeanne decided to search for other proof of a link. She unearthed scholarly animal studies documenting a possible chip-cancer link and posted several of these at the website that she formed as a tribute to Léon:
http://www.noble-leon.com/resourcesAdvanced
Jeanne informed us of this research and even faxed us copies of these studies as they were difficult to obtain. Fortunately, mySpychips co-author Dr. Katherine Albrecht had access to the Harvard library and was able to take Jeanne’s work further, analyzing additional studies that seemed to support a cancer-microchip link in animals.
Sometime later, AP Reporter Todd Lewan entered the picture, eager for an exclusive. He used his press credentials to gain further information, tie up the story with a perfect, documented bow, and broadcast it to media outlets around the world. He and Katherine tirelessly pursued the truth that you can now find published in the explosive AP story.
I promised Jeanne that Katherine and I would share the whole story, and that Léon would be remembered for his contribution. Here’s to you, Jeanne and Léon! I’m so sorry it took tragedy for this information to be brought to light. I applaud your tenacity, bravery, and amazing research skills.
– Liz McIntyre
Posted by liz at 2:08 PM | Comments (13)
July 22, 2007
Associated Press article spotlights VeriChip controversy
Hello to CASPIAN members and friends:
The VeriChip battle is heating up! The Associated Press published a feature article today on the human implant controversy that is appearing on newsstands across America:
Chips: High tech aids or tracking tools?
By Todd Lewan, AP National Writer
July 22, 2007
http://seattlepi.nwsource.com/business/1700AP_Chipping_America.html
The article is highlighted on the Drudge Report and is printed in over 200 newspapers and news outlets around the country, including USA Today, Business Week, Forbes, Fox News, and the Washington Post.
Major papers in Houston, Seattle, Denver, San Jose, Charlotte, Chicago, Kansas City, Miami, and more have picked up the story. It has even reached the UK Guardian newspaper and outlets in Canada and Australia. For a partial list, see:
http://news.google.com/news?hl=en&ned=us&ie=UTF-8&scoring=d&q=microchip+lewan&btnG=Search
The article features a full color photo of our anti-chipping protest in West Palm Beach, Florida and a link to our newhttp://www.AntiChips.com website. It also features quotes by me and my Spychips co-author Liz McIntyre, and mentions our book, “Spychips: How major Corporations and Government Plan to Track Your Every Move with RFID.”
I spoke with Todd Lewan, the AP reporter who wrote the story, and told him everything we know about the downsides of the VeriChip Corporation and its dangerous, Big Brother plans to chip the public. (Liz and I have been posting these stories on our Spychips.com website for several years at http://www.spychips.com/press.html) . Mr. Lewan independently verified many of our concerns and discussed them in the article.
Back in May after our West Palm Beach protest, I asked you all to be patient, as the truth about the VeriChip would soon be coming out. Now our efforts to alert the public are beginning to bear fruit.
Sit tight. This is just the start of the backlash.
In freedom,
Katherine Albrecht, Ed.D.
============================================================
Dr. Katherine Albrecht
Founder and Director, CASPIAN Consumer Privacy
Host of “Uncovering the Truth”
We the People Radio Network, M-F 10AM-12PM EST
http://www.wtprn.com
Co-author of “SPYCHIPS: How Major Corporations and Government
Plan to Track Your Every Move with RFID”
OUR WEBSITES:
Human Chipping: http://www.AntiChips.com
RFID Tagging: http://www.spychips.com
Shopper Cards: http://www.nocards.org
Boycott Gillette: http://www.BoycottGillette.com
Boycott Tesco: http://www.BoycottTesco.com
Bio online at: http://www.spychips.com/media/katherine-albrecht.html
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
Chip Implants Linked to Animal Tumors
By TODD LEWAN
The Associated Press
Saturday, September 8, 2007; 2:04 PM
— When the U.S. Food and Drug Administration approved implanting microchips in humans, the manufacturer said it would save lives, letting doctors scan the tiny transponders to access patients’ medical records almost instantly. The FDA found “reasonable assurance” the device was safe, and a sub-agency even called it one of 2005’s top “innovative technologies.”
But neither the company nor the regulators publicly mentioned this: A series of veterinary and toxicology studies, dating to the mid-1990s, stated that chip implants had “induced” malignant tumors in some lab mice and rats.
“The transponders were the cause of the tumors,” said Keith Johnson, a retired toxicologic pathologist, explaining in a phone interview the findings of a 1996 study he led at the Dow Chemical Co. in Midland, Mich.
Leading cancer specialists reviewed the research for The Associated Press and, while cautioning that animal test results do not necessarily apply to humans, said the findings troubled them. Some said they would not allow family members to receive implants, and all urged further research before the glass-encased transponders are widely implanted in people.
To date, about 2,000 of the so-called radio frequency identification, or RFID, devices have been implanted in humans worldwide, according to VeriChip Corp. The company, which sees a target market of 45 million Americans for its medical monitoring chips, insists the devices are safe, as does its parent company, Applied Digital Solutions, of Delray Beach, Fla.
“We stand by our implantable products which have been approved by the FDA and/or other U.S. regulatory authorities,” Scott Silverman, VeriChip Corp. chairman and chief executive officer, said in a written response to AP questions.
The company was “not aware of any studies that have resulted in malignant tumors in laboratory rats, mice and certainly not dogs or cats,” but he added that millions of domestic pets have been implanted with microchips, without reports of significant problems.
“In fact, for more than 15 years we have used our encapsulated glass transponders with FDA approved anti-migration caps and received no complaints regarding malignant tumors caused by our product.”
The FDA also stands by its approval of the technology.
Did the agency know of the tumor findings before approving the chip implants? The FDA declined repeated AP requests to specify what studies it reviewed.
The FDA is overseen by the Department of Health and Human Services, which, at the time of VeriChip’s approval, was headed by Tommy Thompson. Two weeks after the device’s approval took effect on Jan. 10, 2005, Thompson left his Cabinet post, and within five months was a board member of VeriChip Corp. and Applied Digital Solutions. He was compensated in cash and stock options.
Thompson, until recently a candidate for the 2008 Republican presidential nomination, says he had no personal relationship with the company as the VeriChip was being evaluated, nor did he play any role in FDA’s approval process of the RFID tag.
“I didn’t even know VeriChip before I stepped down from the Department of Health and Human Services,” he said in a telephone interview.
Also making no mention of the findings on animal tumors was a June report by the ethics committee of the American Medical Association, which touted the benefits of implantable RFID devices.
Had committee members reviewed the literature on cancer in chipped animals?
No, said Dr. Steven Stack, an AMA board member with knowledge of the committee’s review.
Was the AMA aware of the studies?
No, he said.
___
Published in veterinary and toxicology journals between 1996 and 2006, the studies found that lab mice and rats injected with microchips sometimes developed subcutaneous “sarcomas” _ malignant tumors, most of them encasing the implants.
_ A 1998 study in Ridgefield, Conn., of 177 mice reported cancer incidence to be slightly higher than 10 percent _ a result the researchers described as “surprising.”
_ A 2006 study in France detected tumors in 4.1 percent of 1,260 microchipped mice. This was one of six studies in which the scientists did not set out to find microchip-induced cancer but noticed the growths incidentally. They were testing compounds on behalf of chemical and pharmaceutical companies; but they ruled out the compounds as the tumors’ cause. Because researchers only noted the most obvious tumors, the French study said, “These incidences may therefore slightly underestimate the true occurrence” of cancer.
_ In 1997, a study in Germany found cancers in 1 percent of 4,279 chipped mice. The tumors “are clearly due to the implanted microchips,” the authors wrote.
Caveats accompanied the findings. “Blind leaps from the detection of tumors to the prediction of human health risk should be avoided,” one study cautioned. Also, because none of the studies had a control group of animals that did not get chips, the normal rate of tumors cannot be determined and compared to the rate with chips implanted.
Still, after reviewing the research, specialists at some pre-eminent cancer institutions said the findings raised red flags.
“There’s no way in the world, having read this information, that I would have one of those chips implanted in my skin, or in one of my family members,” said Dr. Robert Benezra, head of the Cancer Biology Genetics Program at the Memorial Sloan-Kettering Cancer Center in New York.
Before microchips are implanted on a large scale in humans, he said, testing should be done on larger animals, such as dogs or monkeys. “I mean, these are bad diseases. They are life-threatening. And given the preliminary animal data, it looks to me that there’s definitely cause for concern.”
Dr. George Demetri, director of the Center for Sarcoma and Bone Oncology at the Dana-Farber Cancer Institute in Boston, agreed. Even though the tumor incidences were “reasonably small,” in his view, the research underscored “certainly real risks” in RFID implants.
In humans, sarcomas, which strike connective tissues, can range from the highly curable to “tumors that are incredibly aggressive and can kill people in three to six months,” he said.
At the Jackson Laboratory in Maine, a leader in mouse genetics research and the initiation of cancer, Dr. Oded Foreman, a forensic pathologist, also reviewed the studies at the AP’s request.
At first he was skeptical, suggesting that chemicals administered in some of the studies could have caused the cancers and skewed the results. But he took a different view after seeing that control mice, which received no chemicals, also developed the cancers. “That might be a little hint that something real is happening here,” he said. He, too, recommended further study, using mice, dogs or non-human primates.
Dr. Cheryl London, a veterinarian oncologist at Ohio State University, noted: “It’s much easier to cause cancer in mice than it is in people. So it may be that what you’re seeing in mice represents an exaggerated phenomenon of what may occur in people.”
Tens of thousands of dogs have been chipped, she said, and veterinary pathologists haven’t reported outbreaks of related sarcomas in the area of the neck, where canine implants are often done. (Published reports detailing malignant tumors in two chipped dogs turned up in AP’s four-month examination of research on chips and health. In one dog, the researchers said cancer appeared linked to the presence of the embedded chip; in the other, the cancer’s cause was uncertain.)
Nonetheless, London saw a need for a 20-year study of chipped canines “to see if you have a biological effect.” Dr. Chand Khanna, a veterinary oncologist at the National Cancer Institute, also backed such a study, saying current evidence “does suggest some reason to be concerned about tumor formations.”
Meanwhile, the animal study findings should be disclosed to anyone considering a chip implant, the cancer specialists agreed.
To date, however, that hasn’t happened.
___
The product that VeriChip Corp. won approval for use in humans is an electronic capsule the size of two grains of rice. Generally, it is implanted with a syringe into an anesthetized portion of the upper arm.
When prompted by an electromagnetic scanner, the chip transmits a unique code. With the code, hospital staff can go on the Internet and access a patient’s medical profile that is maintained in a database by VeriChip Corp. for an annual fee.
VeriChip Corp., whose parent company has been marketing radio tags for animals for more than a decade, sees an initial market of diabetics and people with heart conditions or Alzheimer’s disease, according to a Securities and Exchange Commission filing.
The company is spending millions to assemble a national network of hospitals equipped to scan chipped patients.
But in its SEC filings, product labels and press releases, VeriChip Corp. has not mentioned the existence of research linking embedded transponders to tumors in test animals.
When the FDA approved the device, it noted some Verichip risks: The capsules could migrate around the body, making them difficult to extract; they might interfere with defibrillators, or be incompatible with MRI scans, causing burns. While also warning that the chips could cause “adverse tissue reaction,” FDA made no reference to malignant growths in animal studies.
Did the agency review literature on microchip implants and animal cancer?
Dr. Katherine Albrecht, a privacy advocate and RFID expert, asked shortly after VeriChip’s approval what evidence the agency had reviewed. When FDA declined to provide information, she filed a Freedom of Information Act request. More than a year later, she received a letter stating there were no documents matching her request.
“The public relies on the FDA to evaluate all the data and make sure the devices it approves are safe,” she says, “but if they’re not doing that, who’s covering our backs?”
Late last year, Albrecht unearthed at the Harvard medical library three studies noting cancerous tumors in some chipped mice and rats, plus a reference in another study to a chipped dog with a tumor. She forwarded them to the AP, which subsequently found three additional mice studies with similar findings, plus another report of a chipped dog with a tumor.
Asked if it had taken these studies into account, the FDA said VeriChip documents were being kept confidential to protect trade secrets. After AP filed a FOIA request, the FDA made available for a phone interview Anthony Watson, who was in charge of the VeriChip approval process.
“At the time we reviewed this, I don’t remember seeing anything like that,” he said of animal studies linking microchips to cancer. A literature search “didn’t turn up anything that would be of concern.”
In general, Watson said, companies are expected to provide safety-and-effectiveness data during the approval process, “even if it’s adverse information.”
Watson added: “The few articles from the literature that did discuss adverse tissue reactions similar to those in the articles you provided, describe the responses as foreign body reactions that are typical of other implantable devices. The balance of the data provided in the submission supported approval of the device.”
Another implantable device could be a pacemaker, and indeed, tumors have in some cases attached to foreign bodies inside humans. But Dr. Neil Lipman, director of the Research Animal Resource Center at Memorial Sloan-Kettering, said it’s not the same. The microchip isn’t like a pacemaker that’s vital to keeping someone alive, he added, “so at this stage, the payoff doesn’t justify the risks.”
Silverman, VeriChip Corp.’s chief executive, disagreed. “Each month pet microchips reunite over 8,000 dogs and cats with their owners,” he said. “We believe the VeriMed Patient Identification System will provide similar positive benefits for at-risk patients who are unable to communicate for themselves in an emergency.”
___
And what of former HHS secretary Thompson?
When asked what role, if any, he played in VeriChip’s approval, Thompson replied: “I had nothing to do with it. And if you look back at my record, you will find that there has never been any improprieties whatsoever.”
FDA’s Watson said: “I have no recollection of him being involved in it at all.” VeriChip Corp. declined comment.
Thompson vigorously campaigned for electronic medical records and healthcare technology both as governor of Wisconsin and at HHS. While in President Bush’s Cabinet, he formed a “medical innovation” task force that worked to partner FDA with companies developing medical information technologies.
At a “Medical Innovation Summit” on Oct. 20, 2004, Lester Crawford, the FDA’s acting commissioner, thanked the secretary for getting the agency “deeply involved in the use of new information technology to help prevent medication error.” One notable example he cited: “the implantable chips and scanners of the VeriChip system our agency approved last week.”
After leaving the Cabinet and joining the company board, Thompson received options on 166,667 shares of VeriChip Corp. stock, and options on an additional 100,000 shares of stock from its parent company, Applied Digital Solutions, according to SEC records. He also received $40,000 in cash in 2005 and again in 2006, the filings show.
The Project on Government Oversight called Thompson’s actions “unacceptable” even though they did not violate what the independent watchdog group calls weak conflict-of-interest laws.
“A decade ago, people would be embarrassed to cash in on their government connections. But now it’s like the Wild West,” said the group’s executive director, Danielle Brian.
Thompson is a partner at Akin Gump Strauss Hauer & Feld LLP, a Washington law firm that was paid $1.2 million for legal services it provided the chip maker in 2005 and 2006, according to SEC filings.
He stepped down as a VeriChip Corp. director in March to seek the GOP presidential nomination, and records show that the company gave his campaign $7,400 before he bowed out of the race in August.
In a TV interview while still on the board, Thompson was explaining the benefits _ and the ease _ of being chipped when an interviewer interrupted:
“I’m sorry, sir. Did you just say you would get one implanted in your arm?”
“Absolutely,” Thompson replied. “Without a doubt.”
“No concerns at all?”
“No.”
But to date, Thompson has yet to be chipped himself.
___
On the Web:
source: http://www.washingtonpost.com/wp-dyn/content/article/2007/09/08/AR2007090800997_pf.html
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
Applied Digital Solutions Makes $30 Million Payment to IBM
http://www.bloomberg.com/apps/news?pid=conewsstory&tkr=ADSX:US&sid=aGdNFkCDmX4g
Credit, LLC, Satisfying All Outstanding Debt Obligations to IBM
Business Editors
PALM BEACH, Fla.–(BUSINESS WIRE)–June 30, 2003–
Payment Improves the Company’s Cash Flow and Liquidity –
Strengthens Net Worth and Shareholder Equity on Balance Sheet
Applied Digital Solutions, Inc. (Nasdaq: ADSX – News) an advanced technology development company, announced today that it has made a $30 million payment to IBM Credit, LLC, the Company’s former senior secured creditor.
Under the Forbearance Agreement with IBM Credit (announced on March 27, 2003), the Company had the right to purchase all of its debt of approximately $95 million (including accrued interest) with a payment of $30 million by June 30, 2003. This $30 million payment, which was finalized today, satisfies in full the Company’s debt obligations to IBM. In 2002, Applied Digital Solutions and Digital Angel Corporation (AMEX: DOC), which is majority owned by the Company, reported consolidated revenues of $99.6 million.
The payment to IBM Credit increases the Company’s cash flow and liquidity. With this payment, the Company has also significantly strengthened shareholder equity on its balance sheet, increased its net worth, and eliminated the negative cash flow for IBM debt payments. The Company also expects to recognize a gain from the settlement of the IBM debt.
Simultaneous with the $30 million payment, the Company completed a $10.5 million, 8.5% convertible debenture transaction with an investor group. The investors can convert the debentures into shares of ADSX’s common stock (subject, in part, to shareholder approval) or shares of common stock the Company already owns in Digital Angel Corporation. The fixed-price conversion feature represents a 5.0% premium based on current ADSX market prices, subject to dilution provisions. Subject to certain conditions, the regular interest (8.5% per annum) and amortization payments for the 29-month debentures (due June 29, 2006) may be made (at the Company’s option) in cash or the shares the Company owns in DOC.
In addition, ADSX has granted warrants to the investors that are exercisable for approximately 5.35 million shares of ADSX’s common stock, or 950,000 of the shares the Company owns in DOC, or a combination of shares from both companies. The exercise prices represent a 15% premium based upon current market prices, subject to dilution provisions. The warrants vest immediately and expire in June 2007.
“This IBM payment represents an important turning point for Applied Digital,” commented Scott R. Silverman, Chairman and CEO. “In the most recent shareholder conference call and in our 2002 annual report, I referred to satisfaction of our debt obligations to IBM Credit as the first pillar of the Company’s foundation for the future. Now that we have strengthened our capital structure, our priorities are to build on this foundation by achieving cash flow positive operating results and revenue growth through sales of our personal safeguard technologies, particularly Digital Angel(TM) and VeriChip(TM).”
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
“VeriChip Corporation’s VeriMed™ Medical Solution Is Now Integrated Into the Hospital Demonstration Area of the IBM Solutions Experience Lab Located in Austin, Texas,” Business Wire, 08 September 2005
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
DIGITAL ANGEL UNVEILED
Human-tracking subdermal implant technology makes debut
Published: 11/01/2000 at 1:00 AM
http://www.wnd.com/2000/11/4324/
A NASDAQ-traded company has finally unveiled its long-touted and
highly controversial “Digital Angel” — a subdermal microchip implant
designed not merely for keeping tabs on pets, but for widespread,
worldwide use in tracking human beings.
The high-tech device, engineered by
Applied Digital Solutions,
Inc. had its debut Monday before an overflow crowd of more than 300 invited guests at Cipriani 42nd Street in New York City.
The audience included U.S. Secretary of Commerce Norman Mineta, who addressed the crowd, as well as other government officials, potential joint-venture/licensing partners and press representatives.
Richard J. Sullivan, Applied Digital Solutions’ chairman and CEO, waxed eloquent about the market potential of Digital Angel, claiming the company has “uncovered a total marketplace that is conservatively estimated to exceed $70 billion.”
Randy Geissler, chairman and CEO of Digital Angel.net Inc., a wholly owned subsidiary, zeroed in on potential applications.
“Our analysis shows that we are well-positioned to move quickly into certain applications while developing a number of others. Two areas of particular interest are in the health-care arena,” he said, “monitoring heart disease and respiratory disease patients.” The tracking and monitoring of pets, he added, is also “right up our alley.”
The demonstration, which was conducted by Dr. Peter Zhou and Dr. Keith Bolton, showed how Digital Angel “can be used to monitor a person’s key body functions — such as temperature and pulse — and transmit that data wirelessly, on a real time basis, along with the accurate location of the person, to a web-enabled ground station or monitoring facility,” according to a press statement.
The technology consists of a miniature sensor device, designed to be implanted just under the skin, that captures and wirelessly transmits the “wearer’s” vital body-function data, such as body temperature or pulse, to an Internet-integrated ground station. In addition, the antenna receives information regarding the location of the individual from the GPS satellite. Both sets of data — medical information and location — are then wirelessly transmitted to the ground station and made available on Web-enabled desktop, laptop or wireless devices.
A more sophisticated version of microchip technologies currently used as electronic ID tags for pets, Digital Angel is powered electromechanically through muscle movement, or it can be activated by an outside monitoring facility.
As WorldNetDaily has reported, in addition to locating missing persons and monitoring physiological data, Digital Angel will be marketed as a means of verifying online consumer identity for the burgeoning e-commerce world.
In August, Sullivan told WND, “We are currently talking to a watch maker who is interested in placing the device on the back of their watches.” He added that “technology is being developed that would allow Digital Angel to function from the back of a cellular phone, transmitting bio-sensor information when carried by the user.”
And in an interview last March, the chief scientist, Zhou, told WorldNetDaily he believes the implant will be as popular as cell phones and vaccines.
Digital Angel “will be a connection from yourself to the electronic world. It will be your guardian, protector. It will bring good things to you,” said Zhou.
“We will be a hybrid of electronic intelligence and our own soul,” he added.
If you’d like to sound off on this issue, please take part in the
Related stories:
Human ID implant to be unveiled soon
Big Brother gets under your skin
Read more at http://www.wnd.com/2000/11/4324/#4DVAzu6AKIJ0jBl9.99
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
RFID – Intelligent transportation system
https://en.wikipedia.org/wiki/Intelligent_transportation_system
https://www.facebook.com/pages/ISOIEC-18000/600377576652986#
ISO/IEC 18000 is an international standard that describes a series of diverse RFID technologies, each using a unique frequency range.
ISO/IEC 18000 consists of the following parts, under the general title Information technology — Radio frequency identification for item management:
- Part 1: Reference architecture and definition of parameters to be standardized
- Part 2: Parameters for air interface communications below 135 kHz
- Part 3: Parameters for air interface communications at 13,56 MHz
- Part 4: Parameters for air interface communications at 2,45 GHz
- Part 6: Parameters for air interface communications at 860 MHz to 960 MHz
- Part 7: Parameters for active air interface communications at 433 MHz
As ISO/IEC 18000-6 is a large document that contains multiple types the document is split and has been published in 2012 as:
- Part 6: Parameters for air interface communications at 860 MHz to 960 MHz General
- Part 61: Parameters for air interface communications at 860 MHz to 960 MHz Type A
- Part 62: Parameters for air interface communications at 860 MHz to 960 MHz Type B
- Part 63: Parameters for air interface communications at 860 MHz to 960 MHz Type C
- Part 64: Parameters for air interface communications at 860 MHz to 960 MHz Type D
The various parts of ISO/IEC 18000 describe air interface communication at different frequencies in order to be able to utilize the different physical behaviors. The various parts of ISO/IEC 18000 are developed by ISO/IEC JTC1 SC31, “Automatic Data Capture Techniques”.
Conformance test methods for the various parts of ISO/IEC 18000 are defined in the corresponding parts of ISO/IEC 18047. (See RFID_testing)
Performance test methods are defined in ISO/IEC 18046. (See RFID_testing)
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
Bob Boyce’s un-requested VeriChip and associated tumor removed
Boyce finally had the second VeriChip implant removed yesterday along with the associated tumor. This time the surgical staff documented the implant with photos, and the surgeon placed the “foreign body” in a specimen container and sealed it to establish chain of custody evidence.
by Sterling D. Allan Pure Energy Systems News Dec 7, 2010 Copyright © 2010
Last year we reported that Bob Boyce, the highly-revered inventor of ultra-efficient electrolysis systems and of a self-charging battery circuit (harnessing energy from the environment, possibly from zero point energy), had contracted terminal cancer and that the originating point was a VeriChip microchip that someone implanted in his right shoulder without his knowledge or permission. He had a chip removed, but it turned out that another chip was still in there, implanted deeper, as confirmed by an X-ray. He’s lived with that one for a year, but finally had it removed yesterday at the Fannin Regional Hospital in Blue Ridge, Georgia.
continue reading this story at :
http://pesn.com/2010/12/07/9501740_Bob_Boyce_verichip_removed/
http://bobboyce.org/cancer.htm
A second VeriChip microchip implant was removed yesterday from Bob Boyce‘s shoulder, which was placed there without his knowledge or consent. The blue color is from a dye that pinpoints cancerous cells, which are not uncommon to form along with VeriChip implants (ref).
The VeriChip “foreign body” was placed in a specimen container and sealed by the surgeon.
part-7-testing-for-radio-frequency-in-the-body
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
PositiveID Corporation Partners With Wireless Technology Leaders
at Connected Development to Complete Final Development of iglucose
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
Marketing RFID using Television Shows & Movies
2002 – The Guardian (CBS) – Kids are tracked by a watch with RFID/GPS
2002 – Law and Order SVU – Child tracked by parents incase kidnapped
2004 – CSI Miami – Night club using implants for payment
2000 – Mission Impossible 2 – Secret Agent tracks girlfriend across the globe
…just to name a few.
Marketing RFID in TV Commercials
If this doesn’t wake people up…
Interac implies that something is “Mobile payments are just the beginning, Imagine whats coming NEXT”
https://www.youtube.com/watch?v=-PFflNdWOZM
RFID Experiments
If they understood the radiation exposure, they likely would not have tested these on humans. Read the links about Dogs with Cancer.
World first bio-payment with BTC
Home made Bio Payment
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
UK : Channel 5 EXPOSED: The Gadget Show’s RFID + Human Microchipping propaganda
If this article is true, most media do not seem to be very interested in finding out more about it :
Trump: We Need a Final Solution to the Immigration Problem
NOVEMBER 13, 2015 by R. HOBBUS J.D. – NEW YORK, Ny
…Trump’s plan includes a “revolutionary” method for locating and tracking individuals who have been or are scheduled to be deported from the United States.
“Specialists will implant a tiny radio-frequency identification (RFID) chip into the upper right arm
of every individual who passes through one of these relocation centers,” Trump said, continuing,
“This allows us to track their movements virtually anywhere in the world, in real-time.”…
http://realnewsrightnow.com/2015/11/trump-we-need-a-final-solution-to-the-immigration-problem/
Trump wants BIOMETRIC System, Tracking System
8/31/2016
” #8, In my administration, We will finally complete the “Biometric Entry Exit Visa System”
and let me tell you, it will be on Land, it will be at Sea, it will be in Air ”
Marty Cooper, Inventor of the first cell phone in 70s …Talks about RFID
5:14 “I envision a future with it embedded under your skin“
2017
Australian ABC writes about Swedish company in Stockholm where 150 employees joined on ‘offer’ free rfid implants. Warning to this harmful technology. Unfortunately, the employees have not been given the whole picture. Wonder what tests have shown that such implants does not cause serious health risks in the long run? Has the evolving industry done the tests themselves?
http://www.abc.net.au/news/2017-04-03/swedish-employees-agree-to-microchip-implants/8410018
Swedish employees agree to free microchip implants designed for office work
April 2, 2017
Would you agree to have a microchip implanted in you by your workplace that could potentially monitor your toilet breaks and how many hours you worked?
Key points:
- The chip is the size of a grain of rice and injected into a person’s hand
- It allows workers to open doors, use electronic devices, with potential for more
- Data collected could include health, location, hours worked, toilet breaks
A Swedish firm in Stockholm — Epicenter — has offered to inject its staff with microchips for free, and around 150 of the company’s young workforce have so far taken up the offer.
The RFID (radio-frequency identification) chips are roughly the size of a grain of rice, and are implanted using a syringe into the fleshy part of the recipient’s hand.
At the moment the chip gives Epicenter’s workers access to doors and photocopiers, but with the promise that further down the track it will include the ability to pay in the cafe.
Patrick Mesterton, co-founder and chief executive of Epicenter, said the biggest benefit of the script was the convenience.
“It basically simplifies your life,” he said.
“You can do airline fares with it, you can also go to your local gym … So it basically replaces a lot of things you have other communication devices for, whether it be credit cards, or keys, or things like that.”
Mr Mesterton said deciding to put something in your body was a big step, and when he first considered it he asked himself: “Why would I do this?”
“But then on the other hand, I mean, people have been implanting things in their body, like pacemakers and stuff, to control their heart,” he said.
“That’s a way, way more serious thing than having a small chip than can actually communicate with devices.”
Media player: “Space” to play, “M” to mute, “left” and “right” to seek.
Epicenter’s chief experience officer Fredric Kaijser, who is also microchipped, said it was common for people to ask him about it when they first found out he had an implant.
“They all get excited about privacy issues and what that means and so forth,” he said.
Monitoring toilet breaks, work hours, location
Certainly the technology could mean trading off an amount of a person’s privacy in exchange for the convenience it offers.
Ben Libberton, a microbiologist from the Swedish thinktank and research organisation the Karolinska Institute, said the data that could be accessed from the embedded chip was very different from the data found on a person’s smartphone.
“Conceptually you could get data about your health, you could [get] data about your whereabouts, how often you’re working, how long you’re working, if you’re taking toilet breaks and things like that,” he said.
“All of that data could conceivably be collected.
“So then the questions is: What happens to it afterwards? What is it used for? Who is going to be using it? Who is going to be seeing it?”
Sandra Haglof, who works for the Stockholm-based event company Eventomatic, said she chose to get the chip because she wanted to be “part of the future”.
“I usually lose a lot of things like my keys … so this will give me access and help me a lot more.”
http://www.abc.net.au/news/2017-04-03/swedish-employees-agree-to-microchip-implants/8410018
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
https://www.youtube.com/watch?v=v2Kmx6JbPvs
Swedish commuters are paying for train journeys with a microchip embedded in their HAND
Implants that are hidden underneath the skin double up as a Swedish version of the Oyster card
A SWEDISH train company is letting passengers use microchip implants in their hands to pay for train journeys instead of paper tickets.
Rail operator SJ claims that up to 100 customers are already the system to board trains, using a tiny flash memory drive surgically embedded under their skin.
Swedish commuters are paying for rail journeys with a chip in their hand
To use the service, passengers on the government-run network have to already have a microchip implant, as SJ doesn’t sell the futuristic technology to its customers.
Up to 20,000 people in Sweden already have microchips implanted in their hands – mainly for use at work instead of a plastic ID card to open doors, use printers and pay for food.
SJ Press Officer, Stephen Ray, told Sun Online that the idea was put forward by a tech company in Stockholm called Epicenter, whose staff already have the chips installed and thought it would be convenient to use them for train travel as well.

The ticketing system uses the same NFC – or Near Field Communication – technology as Oyster or Contactless Cards.
Mr Ray admitted that the project hasn’t been without its teething troubles or privacy concerns though.
One early flaw in the system meant that while using their company smartphones to validate a customers microchip, rail staff would sometimes be shown a passenger’s LinkedIn profile instead of their SJ ticket info.
Apparently some customers had also programmed their microchips to give out a “digital business card” in the form of their LinkedIn profile when scanned by an NFC enabled smartphone, and this was being sent to the conductor’s phone instead of the customer’s train ticket details.

Mr Ray said that the problem was quickly solved though, adding, “that’s why we call it a trial.”
Peter Dahlqvist, Head of SJ Business Sales said: “SJ is already one of Sweden’s most digital companies, so this new project could be started up very quickly.
“The microchip ticket is a good example of how we are happy to try out new ideas alongside customers and help to force the pace of digital development.”
There are currently no plans to bring the scheme to the UK.
https://www.facebook.com/Channel4News/videos/10154933912806939/
https://www.privateinternetaccess.com/blog/2017/05/train-tickets-rfid-tags-europe/
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
Outlawing microchipping humans not so far-fetched, Nevada senator says
By SANDRA CHEREB REVIEW-JOURNAL CAPITAL BUREAU
February 13, 2017 – 4:14 pm
CARSON CITY — State Sen. Becky Harris said a bill to prohibit forced microchipping of people is not as far-fetched as it might seem, because it happens in some places around the world.
Senate Bill 109 would make it a Class C felony to require someone to be implanted with a radio frequency identifier, such as microchips placed in pets.
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
Laws against criminal uses of Electromagnetic Energy Weapons
(1) The European Union Parliament (1999)
The European Parliament A4-0005/1999 Paragraph 27 calls for a worldwide ban on weapons that might enable “any form” of the “manipulation of human beings”.
http://peacepink.ning.com/profiles/blogs/european-parliament-a40005…
2) H.R. 1160 (2001)
Introduced March 22, 2001: terminate operation of the Extremely Low Frequency band
http://peacepink.ning.com/profiles/blogs/house-bill-hr-1160-2001
(3) H.R. 2977 (2001) Introduced by Rep. Dennis Kucinich: peaceful uses of space; prohibiting (the unlawful use) of electromagnetic weapons
http://peacepink.ning.com/profiles/blogs/usa-bill-hr-2977-ih
(4) The Human Rights org (2002)
Media Guide to Disarmament: electromagmentic resonance weapons
The United Nations Institute for Disarmament Research (UNIDIR) formally listed a special category of psychotronic [psycho-“mind” & tronic=”electronic”] mind control and other electromagmentic resonance weapons in their 2002 Media Guide to Disarmament.
http://www.unidir.ch/pdf/activites/pdf2-act201.pdf
(5) Berkeley, California (2002) Ban the weaponization of space and mind control
http://peacepink.ning.com/profiles/blogs/berkeley-resolution-of-ban…
(6) Michigan: House Bill 4513 (2003) Classify harmful electronic or electromagnetic devices
http://peacepink.ning.com/profiles/blogs/house-bill-4513
(7) Michigan: House Bill 4514 (2003) Add to…
View original post 810 more words
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
Tech company workers agree to have microchips implanted in their hands
By ABC News – ENJOLI FRANCIS REBECCA JARVIS
Some workers at a company in Wisconsin will soon be getting microchips in order to enter the office, log into computers and even buy a snack or two with just a swipe of a hand.
Todd Westby, the CEO of tech company Three Square Market, told ABC News today that of the 80 employees at the company’s River Falls headquarters, more than 50 agreed to get implants. He said that participation was not required.
The microchip uses radio frequency identification (RFID) technology and was approved by the Food and Drug Administration in 2004. The chip is the size of a grain of rice and will be placed between a thumb and forefinger.
Swedish company implants microchips in employees
http://abcnews.go.com/GMA/video/swedish-company-implants-microchips-employees-46590108
Westby said that when his team was approached with the idea, there was some reluctance mixed with excitement.
But after further conversations and the sharing of more details, the majority of managers were on board, and the company opted to partner with BioHax International to get the microchips.
Westby said the chip is not GPS enabled, does not allow for tracking workers and does not require passwords.
“There’s really nothing to hack in it, because it is encrypted just like credit cards are … The chances of hacking into it are almost nonexistent because it’s not connected to the internet,” he said. “The only way for somebody to get connectivity to it is to basically chop off your hand.”
Three Square Market is footing the bill for the microchips, which cost $300 each, and licensed piercers will be handling the implantations on Aug. 1. Westby said that if workers change their minds, the microchips can be removed, as if taking out a splinter.
He said his wife, young adult children and others will also be getting the microchips next week.
Critics warned that there could be dangers in how the company planned to store, use and protect workers’ information.
Adam Levin, the chairman and founder of CyberScout, which provides identity protection and data risk services, said he would not put a microchip in his body.
“Many things start off with the best of intentions, but sometimes intentions turn,” he said. “We’ve survived thousands of years as a species without being microchipped. Is there any particular need to do it now? … Everyone has a decision to make. That is, how much privacy and security are they willing to trade for convenience?”
Jowan Osterlund of BioHax said implanting people was the next step for electronics.
“I’m certain that this will be the natural way to add another dimension to our everyday life,” he told The Associated Press.
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
24 Hours After First U.S. Company Forces Microchip On Employees, Horrific Side Effect Surfaces
By Amanda Shea
Although we have heard rumors of micro-chipping Americans becoming a reality in this “brave new world” of technology for years, a big Wisconsin company was first to take the plunge into this dangerous territory. With being the first to accept this risk, the company and their apparently pretty dedicated employees also put themselves in the position of finding out for the rest of us what the ramifications of this experimental decision are.
Your personal safety, privacy, and general way of life as you know it is at risk with this intrusive technology that’s now being forced on Americans in what should be a free country. This microscopic chip compromises all that we hold dear and the surfacing “side effects” appear to be worse than anticipated just 24 hours after the first citizens of the United States accepting this as a good idea.
While talk of the introduction of this little chip used to seem like a conversation among a private society of tinfoil hat conspiracy theorists, until yesterday, this terrifying reality now coming to a workplace near you. Before you’re sold on the idea of convenience and a new era of technology, you need to know what’s been revealed in the last few hours since the inception of this surgically implanted tracking device. More importantly, it needs to be stopped before it takes off even more than it seems to already have.
The Wisconsin-based company who was the first to take this idea and make the chip has announced plans to expand and get the technology out to other companies for “convenient” implants to be used on their employees. This is especially the case for certain companies with high-security levels where fraud is likely. The implants, which are basically tracking devices to void your constitutional right to privacy, are inserted in the webbing of the hand between the thumb and index finger. They are designed this way to allegedly make it easier to get through doors and other security check points that would typically require some sort of ID or card.
“The fun part comes in where they promise that these chips aren’t in any way GPS enabled and can’t be used to read information, only hold information that you might need to use, like using multiple magnetic strips with the swipe of a hand,” Freedom Daily first reported about the introduction of this big idea. “And they will no doubt notify us right away when they decide to GPS enable these chips because big brother never does anything underhanded and keeps it a secret,” the article added, pointing out the obvious privacy issues for the employee, using snark.
Now that the technology is available and presented to the masses in America to use, it only took a few short hours for companies across the country to eagerly get on board with this intrusion. The immediate side effect of this technology was the warning that it’s now going to be a regular part of society, with the projection that by the end of 2017, every American will have one of these in their hand.
“Americans will receive a microchip implant by the end of 2017, NBC warns,” Health Fit Point reports. “With the help of these microchips authorities will be able to identify the person in a matter of milliseconds.”
“According to NBC, this revolution will cause major concern, because people will ask themselves if they really are who they say they are,” the report continued.
“Other people’s concern is that with this upgraded and revolutionized RFID microchip, the federal government will become even more influential because they could see and monitor our every move. In some states like Virginia, there is legislation process against this. The people from NBC are adamant in their claims that the production process of RFID Brain microchip is now over and it is being tested on humans at the moment.”
Many may wonder how this could possibly be constitutional in a free country like America. Just with all gross government overreach, we can thank Barack Obama’s time in office for trampling on our rights to privacy since House Bill H.R. 4872, under the National Medical Device Registry on page 1014, allowed it as an implantable Class II device.
“The RFID microchip allows the government to see our motion, control our food and manage our money. Some experts even speculate that this incredibly small device can kill the person which carries it,” according to Health Fit Point.
This slipped through the government by being buried over 1000 pages into a bill, which was likely intentional. It’s stalker level 2000 to put something in people’s bodies and watch, study, and use everything they do. “You might think that it’s just not possible that they require things like this of us, but if you’ll recall things like drivers licenses and social security numbers weren’t required at one time, but it’s basically impossible to function without them now,” we previously pointed out. “Maybe they won’t be able to pass a law that straps your hand down and implants you, but if big brother wants to track you with an implant, trust me, they’ll make it to where you are completely incapacitated without one.”
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
Animal Microchips Linked To Causing Cancer
Published on May 22, 2017 in Overall Health by Get Holistic Health
Many veterinarians recommend them, and most animal shelters require them. Identification microchips injected into the necks of cats and dogs are touted as useful in recovering lost pets because the devices store owner and medical information. But are they safe? A new lawsuit against Merck & Co., Inc., maker of the HomeAgain pet microchip, says they are not, noting that they can cause cancer to develop in pets.
Featured at www.ChipMeNot.org, a website launched to raise awareness about the harm caused to animals by microchips, the lawsuit alleges that Merck’s HomeAgain pet microchip induces cancerous tumors in pets. According to the suit, the defendant’s cat developed cancer after getting a chip implant, and according to reports, other animals have gotten cancer after getting chipped as well.
“Based on the alarming number of microchip-induced cancers we’re discovering, I predict this lawsuit will be just the tip of the iceberg,” said Dr. Katherine Albrecht, a consumer advocate and expert on side effects associated with implantable microchips. “Merck and organizations that advocate pet chipping should take this lawsuit seriously and start warning pet owners of the risk of microchip-induced cancer.”
According to the U.S. Food and Drug Administration, potential health risks associated with implantable microchips include “adverse tissue reaction”. Based on data from the British Small Animal Veterinary Association, this can include “swelling”, “infection”, “abscesses”, and “tumors”.
Albrecht presented a paper on the subject called “Microchip-Induced Tumors in Laboratory Rodents and Dogs: A Review of the Literature 1990-2006” (http://www.chipmenot.org/pdfs/P074.pdf) at the June conference of the Institute of Electrical and Electronics Engineers that documents the increasing number of animals being harmed by microchips. Currently, there is no repository of data on adverse events associated with microchips in the U.S., but Albrecht organization, CASPIAN, is filling that void by compiling such information and making it available to the public.
To learn more about the dangers of animal microchips, visit: www.ChipMeNot.org
Sources for this story include:
http://www.chipmenot.org/mercksued.htm
http://www.getholistichealth.com/48231/animal-microchips-linked-to-causing-cancer/
Documentary from around the world
https://www.youtube.com/watch?v=k6T1QktPuG0
Olympic Association CEO Proposal to Microchip Athletes “Like Dogs” Spawns CAMCAT
A proposal by a World Olympians Association official that athletes be chipped and tracked like dogs to prevent drug cheats prompts consumer privacy expert Liz McIntyre to form CAMCAT – Citizens Against Marking, Chipping and Tracking.

A proposal by a World Olympians Association official that athletes be chipped and tracked like dogs to prevent “drug cheats” has crossed the line, said consumer privacy expert Liz McIntyre.
McIntyre, co-author of Spychips, a bestseller about the dangers of RFID tracking technology, is forming CAMCAT (Citizens Against Marking, Chipping and Tracking) in response, to combat attempts to chip humans forcibly or by threat of losing benefits.
According to The Guardian newspaper, World Olympians Association CEO Mike Miller made the controversial remarks about human chipping at a recent anti-doping forum in London, suggesting that elite athletes would have to accept implants or drop out of Olympic-level competition:
“In order to stop doping we need to chip our athletes…. Some people say it’s an invasion of privacy, well, sport is a club and people don’t have to join the club if they don’t want to, if they can’t follow the rules,” Miller was quoted as saying.
“We need legislation that guarantees citizens the right to reject tracking implants without fear of losing the right to work or enjoy other pursuits.” ~ Liz McIntyre
“When someone in Miller’s position has the audacity to suggest that RFID dog tracking chips are an acceptable prerequisite for participation in any endeavor, it’s time for action,” McIntyre cautioned. “No human being should ever be forced to accept a tracking implant to fully participate in society.”
While Miller did not spell out what microchip technology he wants to implant in Olympic hopefuls, there are implantable RFID microchips with biosensors that can detect a human or other animal’s health status, diseases and substances in the blood. A chip/biosensor combo could theoretically monitor an athlete’s blood 24/7 and report aberrations when queried by a nearby reader device — perhaps a phone with a built-in reader, for example.
McIntyre warned that chipping elite athletes would pave the way for finding excuses to chip everyone.
“We need legislation that guarantees citizens the right to reject tracking implants without fear of losing the right to work or enjoy other pursuits,” said McIntyre. “CEO Miller’s outrageous recommendation made me realize that we are running out of time. That’s why I formed CAMCAT. Lawmakers need to act now to protect their constituents. CAMCAT will work to make that happen.”
Press Mentions:
Track this! Olympians now face implanted chips
By Bob Unruh, World Net Daily, October 2017
http://www.wnd.com/2017/10/track-this-olympians-now-face-implanted-chips/
How Does the RFID Implant Revolution Affect You?
By Ray Walsh, BestVPN, October 2017
https://www.bestvpn.com/rfid-chip-digital-privacy/
Olympic Athletes Could Soon Face Being Microchipped ‘Like Dogs’ To Compete
By Mark Slavo, Activist Post, October 2017
https://www.activistpost.com/2017/10/olympic-athletes-soon-face-microchipped-like-dogs-compete.html
article source : http://camcat.org/2017/10/17/olympic-association-ceo-proposal-to-microchip-athletes-like-dogs-spawns-camcat/
2018
Scientist Believes the Human Microchip Will Become “Not Optional”
Technologies designed specifically to track and monitor human beings have been in development for at least two decades.
In the virtual realm, software programs are now capable of watching us in real time, going so far as to make predictions about our future behaviors and sending alerts to the appropriate monitoring station depending on how a computer algorithm flags your activities. That is in and of itself a scary proposition.
What may be even scarier, however, is what’s happening in the physical realm. According to researches working on human-embedded microchips it’s only a matter of time before these systems achieve widespread acceptance.
“Chances are you’re carrying a couple of RFID microchips now. And if you are, they’re sending out a 15-digit number that identifies you. That number can be picked up by what’s called an ISO compliant scanner. And they’re everywhere, too. […]
“It’s not possible to interact with society in a meaningful way by not having a mobile phone. I think human implants are likely to go along a very similar route. It would be such a disadvantage to not have the implant that it essentially becomes not optional.”
Video Report:
Your initial reaction to this idea may be one of disbelief. There’s no way society would accept such a device. Why would anyone want to implant this in their body?
Consider for a moment where we are right now. For decades Americans rejected the notion that they would submit to being tracked or recorded.
Yet, just about every American now carries a mobile phone. They’re so prevalent, in fact, that many consider it a “right,” prompting the government to actually provide subsidies to those who can’t afford one on their own.
Embedded in every one of those phones is an RFID chip that can track our every movement via GPS or cell tower triangulation. Moreover, those microphones and cameras that come standard on every phone can be remotely activated by law enforcement surveillance systems, a capability that has existed since the early 2000′s.
But as intrusive as these devices are, they are accepted as the norm by billions of people world wide. Not only that, but no one had to “force” them on us. We are, it seems, the masters of our own enslavement. And we pay top dollar to have the best tracking device money can buy!
Granted, one can simply disconnect from “the grid” by throwing away their cell phone. But, the direction these new monitoring technologies are moving coupled with continued government expansion of surveillance suggests that microchip RFID technology will eventually be non-voluntary.
Michael Snyder of The Truth Wins asks What will you do when you can no longer buy or sell without submitting to biometric identification?
“This technology is going to keep spreading, and it is going to become harder and harder to avoid it. And it is easy to imagine what a tyrannical government could do with this kind of technology. If it wanted to, it could use it to literally track the movements and behavior of everyone. […]
“And one day, this kind of technology will likely be so pervasive that you won’t be able to open a bank account, get a credit card or even buy anything without having either your hand or your face scanned first.”
It’s difficult to imagine a populace that will freely submit to such digital bondage. But as has been the case with the degradation of personal privacy and rights in America, be assured it won’t simply become law over night.
First, the technologies will need to be generally accepted by society. It’ll start with real-time consumer based products like Google Glass.
The older generations may reject it, but in a couple of years you can bet that tens of millions of kids, teens and younger adults will be roaming the streets while sporting cool shades, interactive web surfing and the capability to record everything around them and upload it to the internet instantly.
Next, as we’re already seeing from early adopters, RFID chips will be voluntarily implanted under our skin for everything from access to high security buildings to grocery store purchases.
Eventually, once the concept is generally accepted by the majority, it will become our new “social security number.”
To gain access to official services, you’ll need to be a verified human. Without verification you won’t even be able to purchase a six pack of beer, let alone get medical care or a driver’s license.
Whether we like it or not this is the future. Every purchase you make and every step you take will be tracked by a tiny 15-digit passive microchip, meaning that the only way to “turn it off” will be to physically remove it from your body.
In essence, we’ll soon live in a world of Always On Monitoring.
Our children and grandchildren – at least most of them – will likely not only submit to implantation, they’ll gladly pay the costs so that they, too, can “interact with society in a meaningful way.”
Source humansarefree http://humansarefree.com/2014/04/scientist-believes-human-microchip-will.html
http://www.unseen-pedia.com/scientist-believes-human-microchip-will-become-not-optional/
Alarm over talks to implant UK employees with microchips
Trades Union Congress concerned over tech being used to control and micromanage
Julia Kollewe Sun 11 Nov 2018 18.53 GMT Last modified on Sun 11 Nov 2018 21.43 GMT
Jowan Österlund from Biohax holds a microchip implant the size of a grain of rice between his thumb and forefinger.
Photograph: James Brooks/AP
Britain’s biggest employer organisation and main trade union body have sounded the alarm over the prospect of British companies implanting staff with microchips to improve security.
UK firm BioTeq, which offers the implants to businesses and individuals, has already fitted 150 implants in the UK.
The tiny chips, implanted in the flesh between the thumb and forefinger, are similar to those for pets. They enable people to open their front door, access their office or start their car with a wave of their hand, and can also store medical data.
Another company, Biohax of Sweden, also provides human chip implants the size of a grain of rice. It told the Sunday Telegraph (£) that it is in discussions with several British legal and financial firms about fitting their employees with microchips, including one major company with hundreds of thousands of employees.
The CBI, which represents 190,000 UK businesses, voiced concerns about the prospect.
A CBI spokesperson said: “While technology is changing the way we work, this makes for distinctly uncomfortable reading. Firms should be concentrating on rather more immediate priorities and focusing on engaging their employees.”
The TUC is worried that staff could be coerced into being microchipped. Its general secretary Frances O’Grady said: “We know workers are already concerned that some employers are using tech to control and micromanage, whittling away their staff’s right to privacy.
“Microchipping would give bosses even more power and control over their workers. There are obvious risks involved, and employers must not brush them aside, or pressure staff into being chipped.”
Steven Northam, the founder and owner of Hampshire-based BioTeq, told the Guardian that most of its 150 implants have been for individuals, while some financial and engineering firms have also had the chips implanted in their staff.
BioTeq has also implanted them in employees of a bank testing the technology, and has shipped them to Spain, France, Germany, Japan and China.
They cost between £70 and £260 per person. Northam himself and all the directors at BioTeq and one of his other companies, IncuHive, have been microchipped.
Jowan Österlund, the founder of Biohax and a former body piercer, told the Telegraph that his microchips, which cost £150 each, could help financial and legal firms improve security. “These companies have sensitive documents they are dealing with. [The chips] would allow them to set restrictions for whoever.”
Österlund said big companies, with 200,000 employees, could offer this as an opt-in. “If you have a 15% uptake that is still a huge number of people that won’t require a physical ID pass.”
Last year Wisconsin-based Three Square Market partnered with Biohax and became the first company in the US to microchip its employees, on a voluntary basis.
KPMG, one of the big four accountancy firms, said it was not planning to microchip its employees and “would under no circumstances consider doing so”.
Fellow accounting firms EY and PwC also said they would not consider microchipping their employees. Deloitte declined to comment.
Biohax has plans to open an office in London, according to its website. It claims 4,000 people have been microchipped, mostly in Sweden. It is working with the state-owned Swedish rail firm Statens Järnvägar, to allow its passengers to travel via chip implants rather than train tickets. Biohax did not respond to requests for comment.
India : Biometric Data System used prior to RFID System
2019
Mark of the beast? Thousands of Swedes become microchipped
HELSINGBORG, Sweden, July 17, 2019 (LifeSiteNews) – Thousands of people in Sweden are consenting to having microchips placed under their skin to aid them in financial transactions, traveling on the train, and opening locks on doors.
Sweden’s Biohax International has patented a microchip that can be injected into a human hand and used to carry out financial transactions, unlock doors and access information. Jowan Österlund, 38, began his company in 2013 and, according to Fortune magazine, Biohax has now inserted microchips into 4,000 people in Sweden and others from across Europe.
Österlund told Fortune that he believes “millions” more will want to be injected with the chip. Sweden is already almost cashless, and Swedes trust their government too much to think it would use technology against them.
“It is a cultural thing,” the entrepreneur told Fortune. “We have a faster adoption rate (of new technology) in Sweden, and there is probably a higher level of trust in our government than (in) many other countries. We aren’t scared that we will be taken advantage of.”
His chip can carry train tickets, and Sweden’s national railways are entirely “biochip-capable,” Fortune reported. Many of the country’s “Nordic Wellness” gyms are equipped to recognize biochips as passcards, keys, and passwords to personal information. Fans describe the injected chips as more secure than electronic keys and less cumbersome than the conventional metal ones.
The biochip is especially popular with people who are already interested in such body modifications as tattooing and piercings. Österlund himself is heavily tattooed and his first business was a body-piercing company that offered, among other embellishments, “hot-steel skin branding.”
Edwin Black, investigative journalist and author of War against the Weak: Eugenics and America’s Campaign to Create a Master Race, told LifeSiteNews that the new technology could spell the end of personal freedom for whole populations.
“For many years, the privacy community has feared this moment when the implantable chip would become small enough to be injected into the hand,” Black said by phone.
“This means that human behavior can be trackable and controllable by the injector, and if that injector is a government or a corporation under the control of government, we are turning a corner where government will be able to wipe out a whole class of people based on their origin or opposition to policy,” he continued.
Black mentioned China, whom he called “a pioneer of the eugenics of our time,” and its social credit system, which already controls the behavior and movements of its citizens.
“If this technology becomes widely adopted, especially in totalitarian regimes like China, we could see the dreaded transition to an unknown region of human existence,” the author said.
“With A.I. (Artificial Intelligence), anything will be possible,” he added. “A ‘kill’ mechanism could be part of this implantable.”
“In the same way, it can open doors, it can close doors.”
Injectable microchips are currently more commonly used as pet-trackers and to store medical records on vulnerable patients. A number of American states have introduced laws forbidding anyone to require that other human beings be chipped; this prevents employers from forcing their employees to have the device implanted.
Christian commentators have noted that such a chip bears an eerie resemblance to the so-called “mark of the beast” from the Book of Revelation.
States the ancient last book of the Bible: “And he shall make all, both little and great, rich and poor, freemen and bondmen, to have a character in their right hand, or on their foreheads. And that no man might buy or sell, but he that hath the character, or the name of the beast, or the number of his name.”
In this book, St. John writes about a “second beast” of the Apocalypse who would force people to be branded and made it illegal for anyone to participate in commerce without the brand. What this brand might be has been the subject of speculation since the Book of Revelation was written.
In a 2016 talk given at the Rome Life Forum, LifeSiteNews editor-in-chief John Henry Westen said that having “some mark which allows buying and selling is not evil in and of itself,” only if there are some strings attached to getting such a mark that “makes receiving it worthy of eternal damnation.”
Westen speculated that in a not too distant future, law-abiding citizens will only be able to engage in commerce by means of such a chip. He speculated that citizens will eventually only be able to receive such a chip if they first agree to “sign on to some statement” of so-called human rights that would include an acceptence of homosexual ideology, abortion, and other immoral positions. In this way, signing such a statement to receive such a chip would mean a turn toward Satan and away from God.
https://www.lifesitenews.com/news/mark-of-the-beast-thousands-of-swedes-become-microchipped
COULD HUMANS BE INFECTED BY COMPUTER VIRUSES?
KICKER
Release Date 26 May 2010
A scientist at the University of Reading has become the first person in the world to be infected by a computer virus.
Dr Mark Gasson, from the School of Systems Engineering, contaminated a computer chip which had been inserted into his hand as part of research into human enhancement and the potential risks of implantable devices.
These results could have huge implications for implantable computing technologies used medically to improve health, such as heart pacemakers and cochlear implants, and as new applications are found to enhance healthy humans.
Dr Gasson says that as the technology behind these implants develops, they become more vulnerable to computer viruses.
“Our research shows that implantable technology has developed to the point where implants are capable of communicating, storing and manipulating data,” he said. “They are essentially mini computers. This means that, like mainstream computers, they can be infected by viruses and the technology will need to keep pace with this so that implants, including medical devices, can be safely used in the future.”
Dr Gasson will present his results next month at the IEEE International Symposium on Technology and Society in Australia, which he is also chairing.
A high-end Radio Frequency Identification (RFID) chip was implanted into Dr Gasson’s left hand last year. Less sophisticated RFID technology is used in shop security tags to prevent theft and to identify missing pets.
The chip has allowed him secure access to his University building and his mobile phone. It has also enabled him to be tracked and profiled. Once infected, the chip corrupted the main system used to communicate with it. Should other devices have been connected to the system, the virus would have been passed on.
Dr Gasson said: “By infecting my own implant with a computer virus we have demonstrated how advanced these technologies are becoming and also had a glimpse at the problems of tomorrow.
“Much like people with medical implants, after a year of having the implant, I very much feel that it is part of my body. While it is exciting to be the first person to become infected by a computer virus in this way, I found it a surprisingly violating experience because the implant is so intimately connected to me but the situation is potentially out of my control.
“I believe it is necessary to acknowledge that our next evolutionary step may well mean that we all become part machine as we look to enhance ourselves. Indeed we may find that there are significant social pressures to have implantable technologies, either because it becomes as much of a social norm as say mobile phones, or because we’ll be disadvantaged if we do not. However we must be mindful of the new threats this step brings.”
https://www.reading.ac.uk/news-and-events/releases/PR281590.aspx
http://www.bbc.com/capital/story/20170731-the-surprising-truths-and-myths-about-microchip-implants
Not convinced about YOUR DATA? Then check this out :
https://www.youtube.com/watch?v=Vie1apuAdWg
2020
Netflicks Children’s Cartoon “Smart Mark Zombies” and Cell Towers
ID 2020
ID2020 and partners launch program to provide digital ID with vaccines
Calls for a US ‘digital dollar’ rise
as China powers ahead with a digital yuan
- A growing number of voices are calling for the U.S. to issue a “digital dollar” as China continues to work on a digital version of its currency.
- Christopher Giancarlo, former chairman of the Commodity Futures Trading Commission (CFTC), set up an organization to look into the framework and potential of a digital dollar.
- China’s central bank has said that it is working on a digital yuan.
DAVOS, Switzerland — A growing number of voices are calling for the U.S. to issue a “digital dollar” as China continues to work on a digital version of its own currency.
Users of the U.S. dollar are “underserved by an analogue currency in a digital world,” Christopher Giancarlo, former chairman of the Commodity Futures Trading Commission (CFTC), said during an event in Davos.
“I think it’s very much worth considering,” Neha Narula, director of the Digital Currency Initiative at the Massachusetts Institute of Technology (MIT), told CNBC at Davos.
The comments come as China is working on a digital yuan which some experts believe could come this year.
Why digital currencies?
It’s unclear what form a central bank issued digital currency could take. It could be built on blockchain-like architecture. Blockchain is the underlying technology behind the cryptocurrency bitcoin. But it doesn’t necessarily need to be.
The promise of a central bank digital currency (CBDC) is that it could make cross-border movement of money easier and improve traceability to fight corruption or money laundering, according to Henri Arslanian, global crypto leader at PwC.
“The potential traceability features of CBDCs could, for the first time, give us a good fighting change against corruption and money laundering. CBDCs also could allow policymakers to measure the impact of certain policies accurately and immediately,” Arslanian told CNBC at Davos.
Digital dollar
Giancarlo, who has been dubbed by the media as “Crypto Dad” for his open views to blockchain technology while at the CFTC, advocated that the U.S. issue a digital dollar.
He recently set up the Digital Dollar Foundation along with Accenture to work on the design and potential framework for a digital dollar.
“If it (U.S. dollar) remains an analogue currency the challenges of using it in a digital world are very complex,” he added.
“What we are proposing is a digital form (of that dollar) that would be minted by the central bank … And it would be made available to users through the traditional banking system and other banking enterprises,” Giancarlo said.
Explaining a bit more about his vision and privacy issues, Giancarlo said the digital dollar could be “set up in a way that … (the) federal government doesn’t have the ability to check if you’re shopping at Target or Selfridges.”
MIT’s Narula said the U.S. Federal Reserve needs to boost its knowledge of digital currencies.
“I think the Fed needs to gain expertise in this space. They cannot rely on the private sector for expertise, they need to build their own in-house expertise,” Narula said.
Federal Reserve Chairman Jerome Powell acknowledged that the central bank is looking into the possibilities of a CBDC. While the Fed hasn’t developed its own digital currency, it is continuing to “carefully analyze the costs and benefits of pursuing such an initiative in the U.S.,” Powell said in a letter to lawmakers in November.
Digital dollar timeline
The digital dollar could be 10 to 15 years away, according to Jeff Schumacher, CEO of 55 Foundry, a company incubator and investor.
“The advantaged position of the dollar as the global reserve currency is very powerful and the Fed wants to protect that position. A digital dollar may introduce volatility to the dollar which is undesirable in the short term,” Schumacher told CNBC.
“The U.S. will likely not be the first mover on a centralized digital currency, but will want to have the tech ready once the kinks and pitfalls have been worked out by someone else,” he added.
Giancarlo echoed that view. “The U.S. will not be first nor does it need to be, but it does need to get it right,” Giancarlo said.
China’s push
The People’s Bank of China (PBOC) has said that it’s working on a digital version of the yuan, but has not given a timeline about when it could be issued.
Some experts say that a digital yuan could help China’s currency internationalize and challenge the dominance of the U.S. dollar.
“Ultimately I expect the digital yuan to play an important strategic role in China’s ongoing efforts to become a global financial superpower and compete with the U.S. dollar as the world’s number one reserve currency,” Garrick Hileman, head of research at Blockchain and researcher at the London School of Economics, told CNBC in a recent interview.
For example, the digital yuan could be used in trade and infrastructure deals along China’s Belt and Road Initiative.
Central banks eye digital currencies
China and the U.S. are not the only countries looking at issuing their own digital currencies.
The central banks of Britain, Japan, the euro zone, Sweden and Switzerland have grouped up with the Bank for International Settlements (BIS) to assess potential use cases for such currencies.
CBDC’s have gained momentum recently as central banks look to innovate in the face of competition from China and Facebook’s digital currency called libra.
But the Facebook-led project has faced intense regulatory pushback, with central bankers from Powell to European Central Bank Board Member Benoit Coeure warning on the potential risks of libra to global financial stability.
“The future of money is a big topic being discussed this week in Davos. And libra and China’s DCEP (digital currency) have probably been the catalysts that have brought this topic on the top of the agenda,” Arslanian said.
—CNBC’s Ryan Browne contributed to this report.
‘Digital dollar’ proposal stripped from latest Congressional coronavirus stimulus bill
A review of the latest version of House Democrats’ coronavirus economic stimulus bill shows that language around a proposed “digital dollar” has been removed.
As The Block reported on Monday, a version of the bill circulating this weekend included the noteworthy proposal, which would have – if passed – created a system of digital dollar wallets maintained and operated by Federal Reserve system banks. The purpose: to serve as the infrastructure for delivering stimulus payments to American consumers as they weather the economic storm triggered by the spread of COVID-19, the disease caused by the coronavirus pandemic.
A separate bill, previously advanced by members of the House Financial Services Committee, still includes the digital dollar provision.
Yet as The Block noted yesterday, the digital dollar proposal wasn’t guaranteed to survive the political back-and-forth around the bill. A source with knowledge of the process on Capitol Hill had pointed to the problem of setting up such a system from the ground up.
The sprawling Take Responsibility for Workers and Families Act, which now weighs in at more than 1,400 pages, makes no reference to the digital dollar. The payout method has also been significantly altered, appearing to take the form of an advance tax refund via the Treasury Department.
https://finance.yahoo.com/news/digital-dollar-proposal-stripped-latest-141213745.html
Why Human microchipping is so popular in Sweden – ITV News
June 28, 2019
Microchipping people: Associate Professor Katina Michael at TEDxUWollongong
Human Implants : from invasive to pervasive: Mark Gasson at TEDxGoodenoughCollege
Implants & Technology — The Future of Healthcare? Kevin Warwick at TEDxWarwick
2020
Patent for Cryptocurrency system : Human recognition and Wireless Communication
April 18, 2020
Microsoft Patent 060606 – Body interfaced digital currency
What are the chances a device on the body that is used for digital translations would have a patent number 060606?
Also, Microsoft patented this on March 26th of this year.
The full patent id is WO/2020/060606.
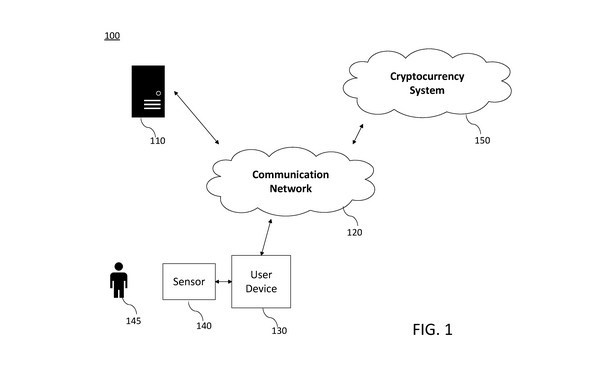
“THE BLACK BOX (COMPUTERS) GIVES THE HUMAN A DEVICE “COUPLED” TO THEIR BODY WITH A NUMBERED CRYPRO CURRENCY WALLET.
THE PERSON IS MONITORED AND COMMUNICATED WITH OVER THE INTERNET VIA THE “COUPLED” DEVICE TO THEIR BODY. A PERSON IS GIVEN CREDITS / MONEY / CRYPTOCURRENCY BASED ON THE BODIES MOVEMENTS (PHYSICAL COMPLIANCE, LABOUR, MOVEMENTS) WITH WHICH YOU CAN THEN USE TO BUY AND SELL….
From the Patent:
“Human body activity associated with a task provided to a user may be used in a mining process of a cryptocurrency system. A server may provide a task to a device of a user which is communicatively coupled to the server.
A sensor communicatively coupled to or comprised in the device of the user may sense body activity of the user.
Body activity data may be generated based on the sensed body activity of the user. The cryptocurrency system communicatively coupled to the device of the user may verify if the body activity data satisfies one or more conditions set by the cryptocurrency system, and award cryptocurrency to the user whose body activity data is verified.”
Okay, this is creepy. Mark of the beast?
But read for yourself.
Here is the link to the patent:
WO2020060606 CRYPTOCURRENCY SYSTEM USING BODY ACTIVITY DATA patentscope.wipo.int
International Patent: wo/2020/060606 * cryptocurrency system using body activity
Applicants: Microsoft Technology Licensing, LLC
Again read this abstract.
Abstract: “Human body activity associated with a task provided to a user may be used in a mining process of a cryptocurrency system. A server may provide a task to a device of a user which is communicatively coupled to the server. A sensor communicatively coupled to or comprised in the device of the user may sense body activity of the user. Body activity data may be generated based on the sensed body activity of the user. The cryptocurrency system communicatively coupled to the device of the user may verify if the body activity data satisfies one or more conditions set by the cryptocurrency system, and award cryptocurrency to the user whose body activity data is verified.”
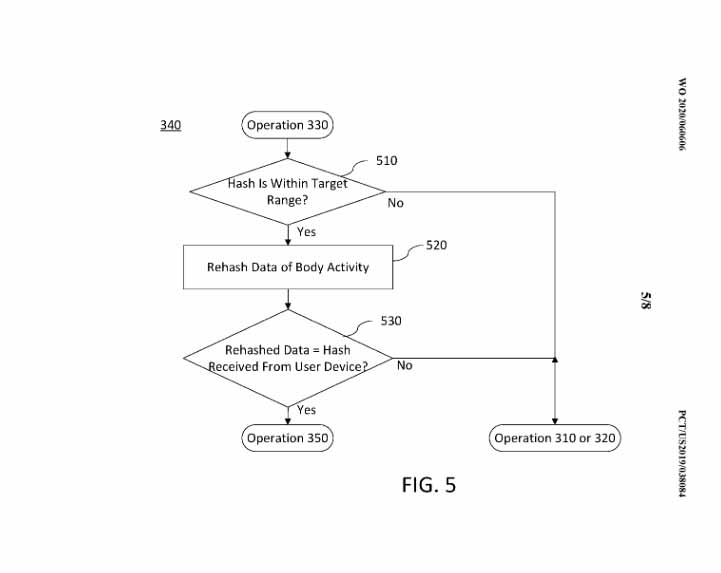
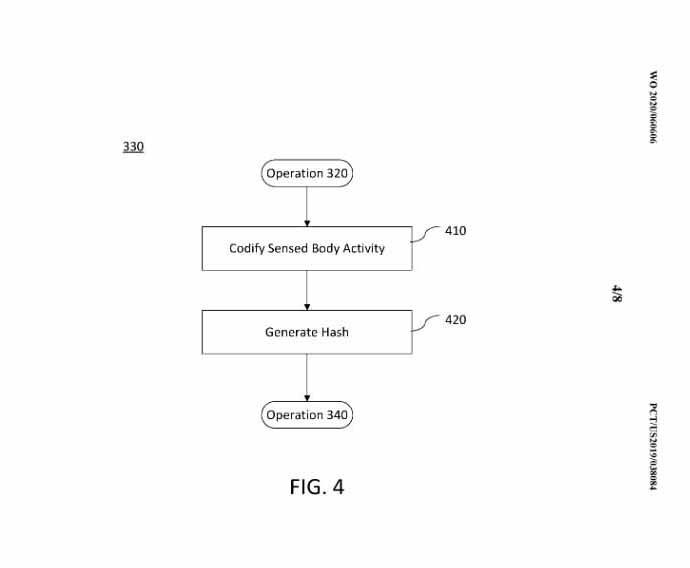
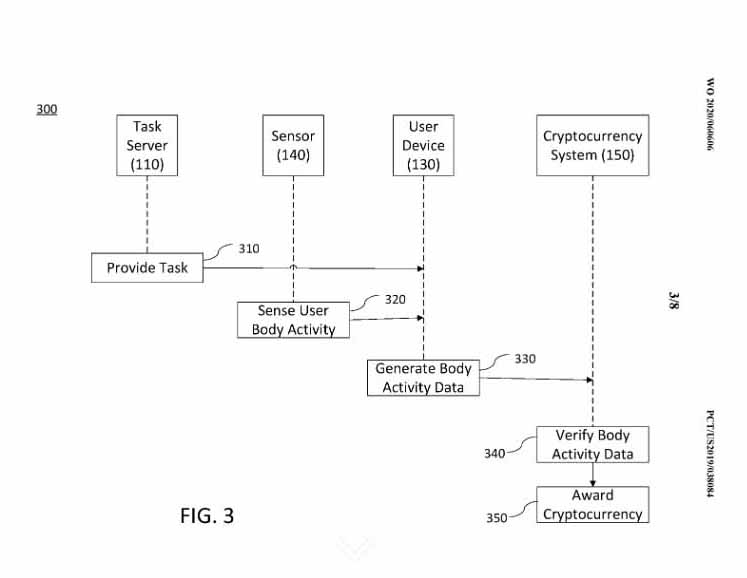
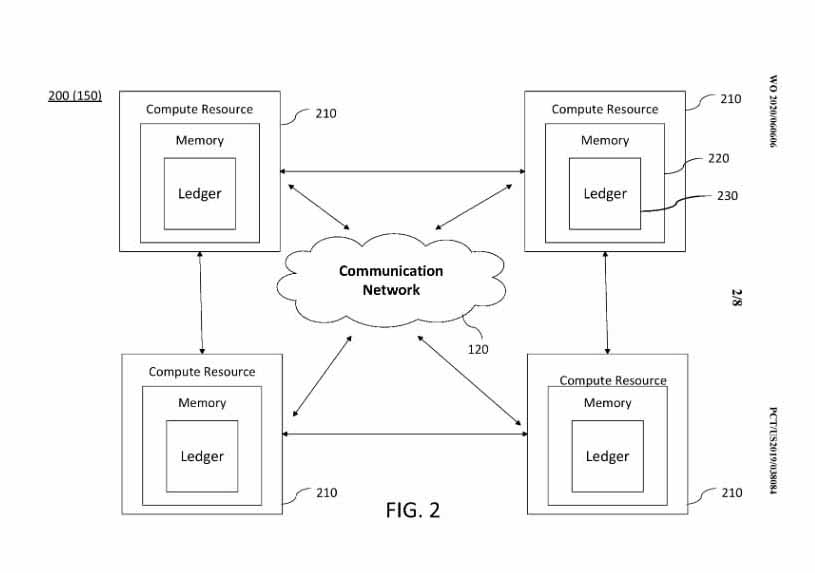
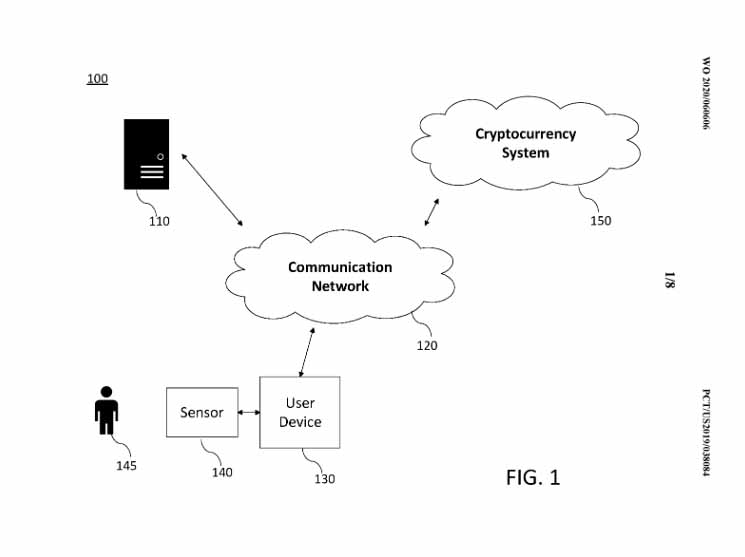


https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020060606&tab=PCTBIBLIO
https://5g-emf.com/operation-corona-microsoft-patent-060606-body-interfaced-digital-currency/
MYPINPAD receives world’s first certification for Contactless Card Payments on mobile devices
UL, a leading global safety science company, today announced that MYPINPAD, a provider of secure personal authentication for payment solutions on smartphones and tablets, is the first company globally to be certified to the Payment Card Industry (PCI) Security Standards Council’s Contactless Payments on COTS (CPoC™) Security and Testing Requirements for its MYPINPAD SoftPOS solution.
A set of principles and requirements for a mobile payment-contactless acceptance solution, CPoC Security and Test Requirements provide a security framework to protect the confidentiality and integrity of sensitive payment information captured and processed in CPoC solutions. MYPINPAD SoftPOS facilitates contactless chip-based payments using the near-field communication (NFC) interface of common commercial off-the-shelf (COTS) devices, such as a smartphones or tablets. Designed to allow merchants payment acceptance flexibility, MYPINPAD SoftPOS addresses online based contactless chip-based transactions that further enables merchant acceptance.
CPoC certification means that MYPINPAD SoftPOS meets key security requirements that help ensure the security of the payment data obtained through the NFC interface and contactless kernel on the COTS device. These security mechanisms, controls and mitigations protect the consumer’s account data and other assets, such as cryptographic keys, helping to ensure the payment process is protected even when performed on consumer grade devices such as mobile phones.
According to Colin Greene, CEO, MYPINPAD, the CPoC certification provides marketplace confidence in MYPINPAD’s technology. “We are extremely excited about our collaboration with UL. With its extensive experience in the payments industry, UL has helped MYPINPAD drive trust with both partners and customers and paved the way for significant adoption of a disruptive technology that has the potential to change forever card payment transaction schemes,” said Greene.
Currently, merchants can accept card payment via a dedicated credit card terminal, or process payment through hardware readers that can transfer magnetic stripe data to a payment application running on a PC or mobile device. MYPINPAD SoftPOS allows the merchant to completely bypass both options and conduct a contactless transaction on a smartphone or tablet without any external hardware.
Isabelle Noblanc, vice president and general manager, UL’s Identity Management and Security division, said, “Companies developing smart and secure payment systems that power successful businesses and brands must innovate with security guiding the way. This is why, as the leading safety and security authority, UL applauds MYPINPAD for achieving the first-ever certification for PCI’s CPoC requirements. This milestone helps ensure MYPINPAD compliance and further demonstrates how UL is enhancing payment ecosystem safety.”
About UL
UL helps create a better world by applying science to solve safety, security and sustainability challenges. We empower trust by enabling the safe adoption of innovative new products and technologies. Everyone at UL shares a passion to make the world a safer place. All of our work, from independent research and standards development, to testing and certification, to providing analytical and digital solutions, helps improve global well-being. Businesses, industries, governments, regulatory authorities and the public put their trust in us so they can make smarter decisions. To learn more, visit UL.com. To learn more about our nonprofit activities, visit UL.org.
DOD AND HHS AWARD $138 MILLION CONTRACT TO APIJECT SYSTEMS TO PROVIDE PREFILLED COVID-19 VACCINE SYRINGES WITH RFID MICROCHIP TRACKING SYSTEM
DOD, HHS award $138m contract to ApiJect Systems America as part of a plan to expand U.S. production capability for domestically manufactured, medical-grade injection devices.
Back in March, the Department of Health and Human Services partnered with a company called ApiJect, what does ApiJect they make? They make pre-filled syringes for injecting people with vaccines, and then provide RFID microchip tracking after the shot is administered. You will see in the main graphic for this article an RFID syringe displayed on the screen of a mobile device. Today, May 12, the DoD and the HHS handed ApiJect a check for $138 million, with an order to deliver hundreds of millions of these devices by October of 2020.
Spearheaded by the DOD’s Joint Acquisition Task Force (JATF), in coordination with the HHS Office of the Assistant Secretary for Preparedness and Response, the contract will support “Jumpstart” to create a U.S.-based, high-speed supply chain for prefilled syringes beginning later this year by using well-established Blow-Fill-Seal (BFS) aseptic plastics manufacturing technology, suitable for combatting COVID-19 when a safe and proven vaccine becomes available.
The contract also enables ApiJect Systems America to accelerate the launch of RAPID USA manufactured in new and permanent U.S.-based BFS facilities with the ultimate production goal of over 500 million prefilled syringes (doses) in 2021.
Welcome to the ‘new normal’, it comes with an RFID microchip-enabled COVID-19 vaccination syringe with your name on it. That’s the new normal. The only question is what will you do when they come to your door, and tell you it’s mandatory? You might want to figure out your response to that scenario sooner rather than later. Now would be a good time.
New Public-Private Partnership Created to Develop a U.S.-Based High-Speed, Emergency Surge Drug Packaging Solution, Using Mass-Manufacturable Prefilled Syringes with Optional Mobile-Based GPS Tracking and Confirmation
FROM PRNEWSWIRE: ApiJect Systems America, a public benefit corporation based here, today joined with the U. S. Department of Health and Human Services (HHS) Office of the Assistant Secretary for Preparedness and Response in announcing the launch of a public-private partnership dedicated to creating a U.S.-based high-speed, high-volume, emergency drug packaging solution, establishing “surge capacity” for mass-manufacturable prefilled syringes.
“TODAY THE DEPARTMENT OF DEFENSE AND THE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES, ANNOUNCE A $138 MILLION CONTRACT WITH APIJECT SYSTEMS AMERICA FOR “PROJECT JUMPSTART” AND “RAPID USA,” WHICH TOGETHER WILL DRAMATICALLY EXPAND U.S. PRODUCTION CAPABILITY FOR DOMESTICALLY MANUFACTURED, MEDICAL-GRADE INJECTION DEVICES STARTING BY OCTOBER 2020.” LT. COL. MIKE ANDREWS, DEPARTMENT OF DEFENSE SPOKESMAN READ MORE
The new consortium, called RAPID — Rapid Aseptic Packaging of Injectable Drugs — will be created and managed by ApiJect Systems America. Its purpose is to enable the U.S. Strategic National Stockpile (SNS) to fill and finish hundreds of millions of prefilled syringes to respond quickly and effectively to health emergencies such as COVID-19. The RAPID Consortium will build a surge capacity network of up to eight domestic packaging facilities using a well- established, drug-packaging process called Blow-Fill-Seal (BFS). The BFS process, used in sterile manufacturing facilities worldwide, features a high volume, small medical-grade plastic container that holds a prefilled volume of medicines or vaccines. FDA-approved BFS technology is already used to package billions of doses annually for medicines to treat respiratory conditions, rotavirus oral vaccines and more. The RAPID Consortium will combine this well-established BFS technology with an innovative interlocking needle hub. The result is a prefilled syringe that eliminates the inefficiencies and difficulties of packaging medicines in, and drawing medicines from, glass vials using disposable syringes.
ABOUT APIJECT
ApiJect Systems America is dedicated to making injectable medicines safe and available for everyone. By using high-speed, high-volume Blow-Fill-Seal plastics technology, we can supply hundreds of millions of ultra-low-cost prefilled syringes in 30 days – with optional RFID tags to enable GPS-based mobile tracking. This will enable governments to better defend their citizens against pandemics, while also improving global access to essential medicines. ApiJect Systems America is contracted with the U.S. government to create and manage the Consortium for Rapid Aseptic Packaging of Injectable Drugs (the RAPID Consortium), a public-private partnership. When fully funded and built out, RAPID will give the U.S. Department of Health and Human Services Office of the Assistant Secretary for Preparedness and Response (ASPR), and the U.S. Strategic National Stockpile, the capability to fill and finish up to 330 million prefilled syringes per month to respond quickly and efficiently to national or smaller-scale health emergencies. The BFS prefilled syringe was conceived and developed by ApiJect’s Head of R&D, noted UK public health leader Marc Koska, OBE. Mr. Koska previously innovated the K1 Auto-Disable Syringe, which is estimated to have saved 12 million lives to date by supporting safe injections. Learn more about ApiJect Systems America at www.apiject.com.

A DARPA Funder Implantable BioChip to Detect COVID-19 : Could hit markets by 2021
An experimental new vaccine developed jointly with the US government claims to be able to change human DNA and could be deployed as early as next year through a DARPA-funded, injectable biochip.
Sept 17, 2020 by Raul Diego
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
MNPs – Magnetic nanoparticles
https://www.bitchute.com/video/iaBMjMWDilqg/
“injecting GRAPHENE OXIDE”….
https://www.bitchute.com/video/n4gesWxueup7/
“Graphene, can be applied in (5G) Antenna design”
Graphene is also said to be used for “Biocidal coating on masks”!
from https://www.bitchute.com/video/oOZi1MsP7FL6/
Scientists are saying if you’re magnetic you have 2 years to live. you have been transfected with weaponized chemicell it will progress to full paralysis from acute myelin sheath Nerve damaged associated with a Magnetofection weaponized Guillain-Barre syndrome transfection leading to death.
watch video below and pray.
Magnetofection – Magnetism Added To Mrna Vaccine Intentionally To Force It Round Your Body = https://www.bitchute.com/video/
P8AdDHdTKxxl/
Magnetofection – the new gene transfection technology = http://www.chemicell.com/products/magnetofection/docs/magnetofection_2_4.pdf
Magnetofection – geneMAG-RNA / DNA – http://www.chemicell.com/products/rna_purification/purification_rna.html
Magnetofection – https://en.wikipedia.org/wiki/Magnetofection
Magnetofection – The Use of Magnetic Nanoparticles for Nucleic Acid Delivery = http://cshprotocols.cshlp.org/content/2009/6/pdb.prot5230.abstract
Magnetofection – enhancing and targeting gene delivery by magnetic force in vitro and in vivo = https://www.nature.com/articles/3301624
Magnetofection – Welcome to chemicell! = http://www.chemicell.com/home/index.html
Magnetofection – Magnetofection™ = http://www.chemicell.com/products/Magnetofection/Magnetofection_separation.html
Magnetofection – http://www.chemicell.com/products/purification/docs/geneMAG-RNA-DNA.pdf
Magnetofection – http://www.chemicell.com/products/ferrofluid/ferrofluids.html
Magnetic Vax – Its in the meat = https://www.bitchute.com/video/rYEKYWQbRqC8/
Magnetic Vax – Dr. Pierre Gilbert in 1995 telling us about Magnetic Vaccines being used to control people = https://www.bitchute.com/video/bAqPKSCcdl1o
Fauci’s Genocide exposed by Dr Robert Willner 30 Years ago = https://www.bitchute.com/video/qAvYZhc6YkKc/
Magnetic Vax – Magnetic Particles in vaccine bottle = https://www.bitchute.com/video/iCnZquRJp45z/
Magnetic Vax – Super-paramagnetic nanoparticle delivery of DNA vaccine = https://pubmed.ncbi.nlm.nih.gov/24715289
Magnetic Vax – Vaccinated people now magnetic = https://www.bitchute.com/video/kRiz8vCymyaO
Magneto nano particle in all Jabby Connecting people to internet w/o there consent, Human slavery = https://www.bitchute.com/video/0DQauWFtm7mI/
Man’s Body Entirely Magnetic After 2 Moderna Shots = https://www.bitchute.com/video/YAxzdwA3Xl1z/
WO 2020 060606 – Bill Gates Patent for CRYPTOCURRENCY SYSTEM USING BODY ACTIVITY DATA = https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020060606 “Human body activity associated with a task provided to a user may be used in a mining process of a cryptocurrency system. A server may provide a task to a device of a user which is communicatively coupled to the server.”
The D-Wave 2X™ Quantum Computer Technology Overview = https://www.dwavesys.com/sites/default/files/D-Wave%202X%20Tech%20Collateral_1016F_0.pdf
Vaxxed are Patented and legally property of company that made the vax = https://www.bitchute.com/video/9I7vYxO6xZXB/
Russian hacker finds real time vaccine tracker database body bio transmission information = https://www.bitchute.com/video/zl5b4HjceDfU
Injectable Microchips in vaccine = https://www.bitchute.com/video/CkMqZpShFNOb
Bluetooth compatible vaccine = https://www.bitchute.com/video/KdnaFa0YczYE
World’s Smallest Single-Chip System Injected = https://www.bitchute.com/video/y4n8HYgAjK6B/
The Beast – We Are Being Counted = https://discover.hubpages.com/education/The-Beast-Supercomputer
Dr. James Giordano – Nanotechnology, Mind Control = https://www.bitchute.com/video/8STGacNUfnMR/
Guillain-Barre syndrome = https://www.mayoclinic.org/diseases-conditions/guillain-barre-syndrome/symptoms-causes/syc-20362793
Guillain-Barre syndrome weaponized for depopulation purposes.
Guillain-Barre syndrome
https://www.mayoclinic.org/diseases-conditions/guillain-barre-syndrome/symptoms-causes/syc-20362793
“Guillain-Barre syndrome may also occur after infection with the COVID-19.” Guillain-Barré syndrome (GBS) is a life-threatening polyradiculoneuropathy with a reported mortality rate of 3% to 13%. The most frequently described causes of death in GBS are respiratory failure, pneumonia, cardiac arrest, and autonomic dysfunction.
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
Cisco ( who also supply the Smart Meter routers to track Data for Itron ) have an interest in RFID for tracking products.
ADSX (Applied Digital Solutions) is also interested in Smart Meters
IBM is also part of both the Smart Meter technology and the Implant chip technology
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
tags : IBM, Cisco, Intel, Texas Instruments, Destron Fearing, ADSX, Verichip, VeriTag, PositiveID, Implant, RFID, EM Microelectronics, 5G
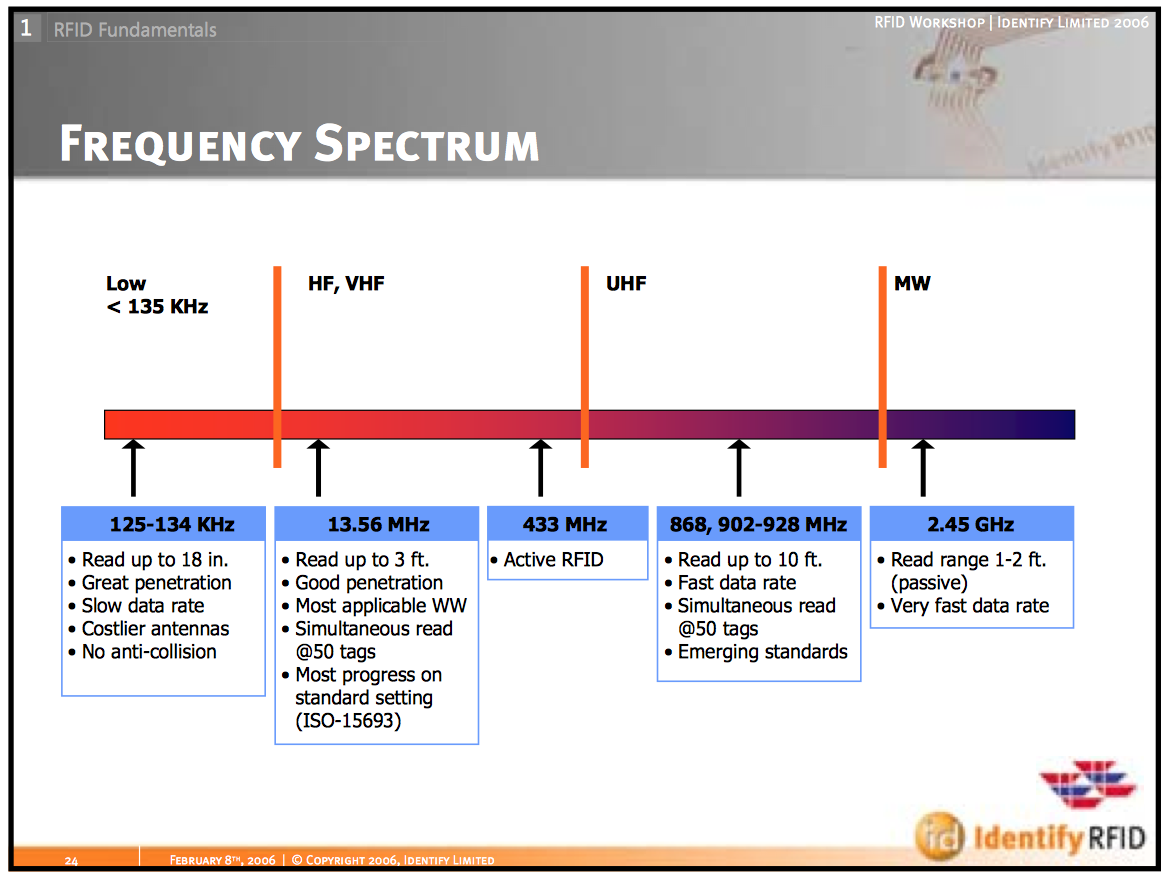
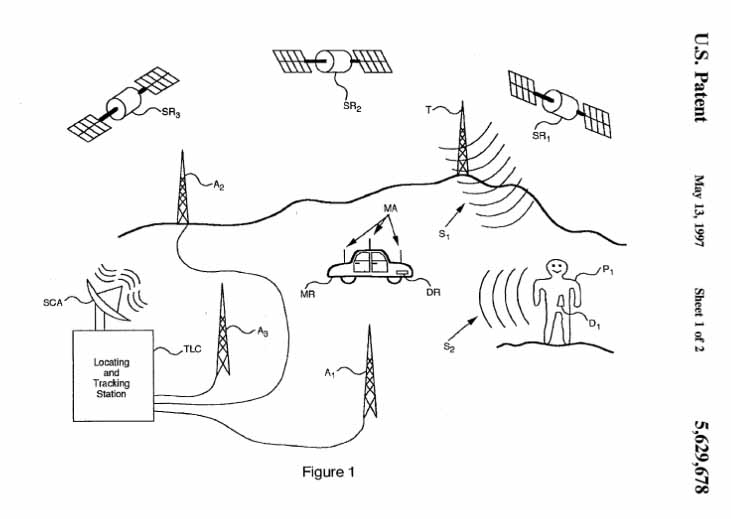




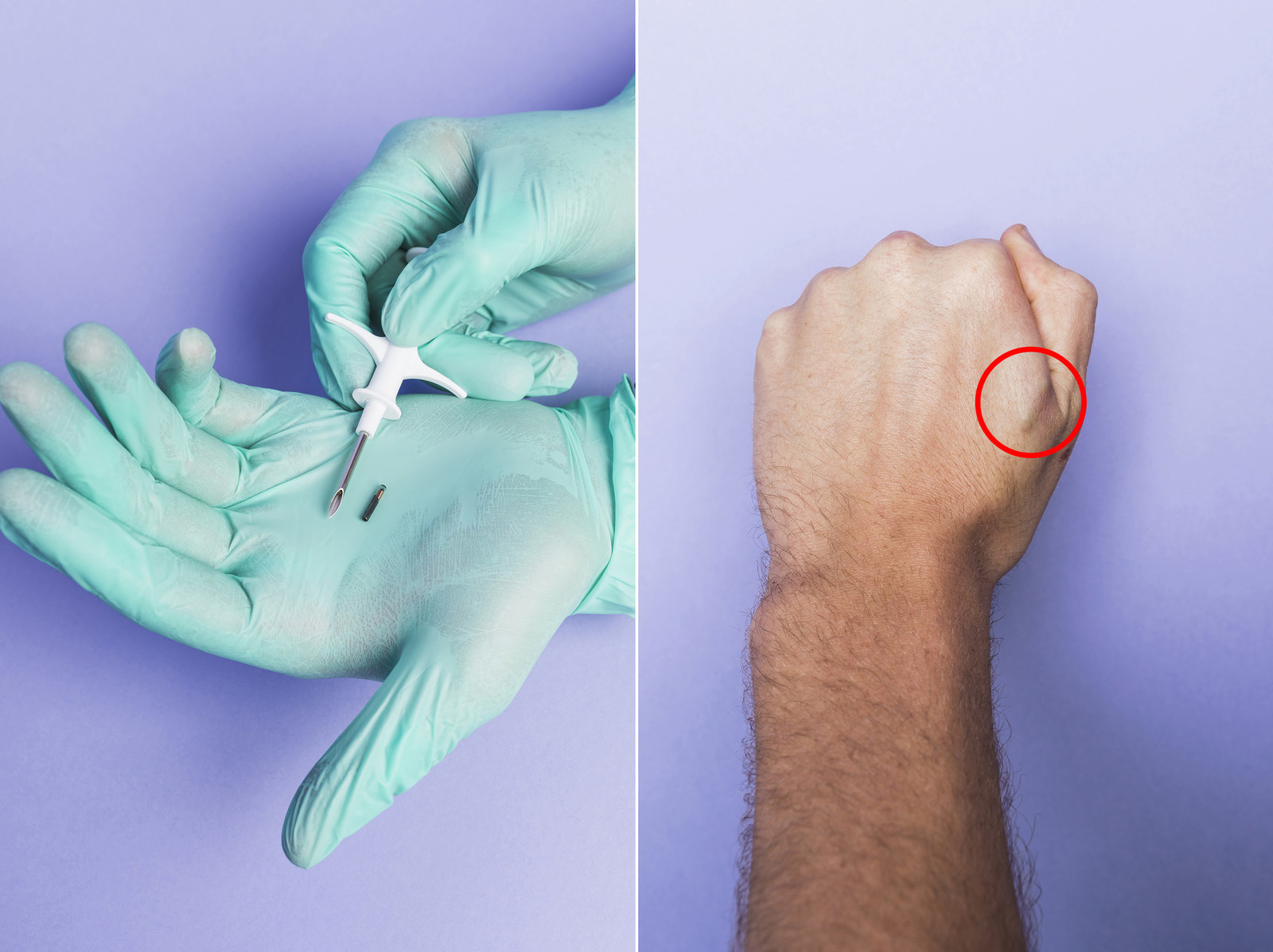






#1 by Donne on November 24, 2015 - 4:15 pm
Quote
I was implanted in 2004 by a surgeon my dentist sent me to who did nothing, but knock me out for 20 minutes and implanted a chip somewhere in my body or mouth, but from that day forward I’ve been living in the most frightening world I’ve ever known, studied like a lab rat from Universities all over the world. They have complete control of all forms of communications, destroy my business, cult like in every way possible. I have never know such evil existed! I can’t express that enough. These implants serve nothing positive for the human race, but they know how to physically and mentally torture their targets and their families and anyone who tries to help them. These people do it all in the name of research. All tax dollars are going to this research that will destroy the human race, our wonderful, not perfect, but still 100% better than being controlled. These are the people that need to be stopped NOW!
Donna
#2 by Walt McGinnis on July 25, 2017 - 3:34 pm
Quote
This is a very thorough analysis of the crisis we are in. Thank you for the fine work, it is top notch.
Pingback: Sweden Is The World’s First Cashless Country, Is Australia Next? « EMR Health Alliance of BC
Pingback: 5G in disguise, with the microchip transhumanism advances (cleared through the front pages of newspapers!) « EMR Health Alliance of BC
Pingback: Trump wants RFID « EMR Health Alliance of BC
Pingback: ID2020 and partners launch program to provide digital ID with vaccines « EMR Health Alliance of BC
Pingback: Amazon Introduces Ability To Pay Using “Palm Recognition Technology” « EMR Health Alliance of BC
Pingback: A DARPA Funder Implantable BioChip to Detect COVID-19 : Could hit markets by 2021 « EMR Health Alliance of BC
#3 by Jeremy C. on March 28, 2023 - 2:09 am
Quote
I have been microchipped for mental illness a while ago. And I have seen people tell me that I have some kind of 5G device in my hand which can carry cellphone data along with a wifi connection.. Yes, it is crazy, until I meet God at heaven’s gates I will never ever bump into people like this again where they do it for research and don’t know that we are being followed and studied like lab rats.